
Acta Horticulturae Sinica ›› 2024, Vol. 51 ›› Issue (9): 2019-2030.doi: 10.16420/j.issn.0513-353x.2023-0629
• Genetic & Breeding · Germplasm Resources · Molecular Biology • Previous Articles Next Articles
REN Siyuan1, CHEN Sen1, LONG Zhijian1, WANG Boya1, TANG Dengguo1, WANG Zhengqian2, YANG Bin3, HU Shanglian1, CAO Ying1,*( )
)
Received:2023-08-29
Revised:2024-07-12
Online:2024-09-25
Published:2024-09-19
Contact:
CAO Ying
REN Siyuan, CHEN Sen, LONG Zhijian, WANG Boya, TANG Dengguo, WANG Zhengqian, YANG Bin, HU Shanglian, CAO Ying. Nitrogen Allocation Characteristics and Expression of Related Genes During Corm-Forming Stage of Amorphophallus konjac[J]. Acta Horticulturae Sinica, 2024, 51(9): 2019-2030.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.ahs.ac.cn/EN/10.16420/j.issn.0513-353x.2023-0629
| 引物名称 Primer name | 引物序列(5′-3′) Primer sequence | 引物名称 Primer name | 引物序列(5′-3′) Primer sequence |
|---|---|---|---|
| AkNIA1-F | GTGTGGTACGTGGTGAATGAG | AkGSR2-1-F | TGGAACTATGATGGATCGAGCA |
| AkNIA1-R | GTTCTTAACGTCGTAGCCCATC | AkGSR2-1-R | TGTGTACTCCTGTTCAATCCCA |
| AkNIR1-F | GAAGGTGAAGCTGGAGAAGGAG | AkASN1-F | AGAGAATTTCGTGGACATGCTG |
| AkNIR1-R | CTTCAGCCTCATCATGAACCG | AkASN1-R | AAATGCTCGCAGTCATCATTGA |
| AkGLU1-F | GTTGTCCTTGGAAAAGTGGGAA | Tubulin-F | GCCGTGAATCTCATCCCCTT |
| AkGLU1-R | ATCCTGCCCATCACAAATTCTG | Tubulin-R | TTGTTCTTGGCATCCCACAT |
Table 1 The qRT-PCR primer sequences of nitrogen metabolism-related genes
| 引物名称 Primer name | 引物序列(5′-3′) Primer sequence | 引物名称 Primer name | 引物序列(5′-3′) Primer sequence |
|---|---|---|---|
| AkNIA1-F | GTGTGGTACGTGGTGAATGAG | AkGSR2-1-F | TGGAACTATGATGGATCGAGCA |
| AkNIA1-R | GTTCTTAACGTCGTAGCCCATC | AkGSR2-1-R | TGTGTACTCCTGTTCAATCCCA |
| AkNIR1-F | GAAGGTGAAGCTGGAGAAGGAG | AkASN1-F | AGAGAATTTCGTGGACATGCTG |
| AkNIR1-R | CTTCAGCCTCATCATGAACCG | AkASN1-R | AAATGCTCGCAGTCATCATTGA |
| AkGLU1-F | GTTGTCCTTGGAAAAGTGGGAA | Tubulin-F | GCCGTGAATCTCATCCCCTT |
| AkGLU1-R | ATCCTGCCCATCACAAATTCTG | Tubulin-R | TTGTTCTTGGCATCCCACAT |
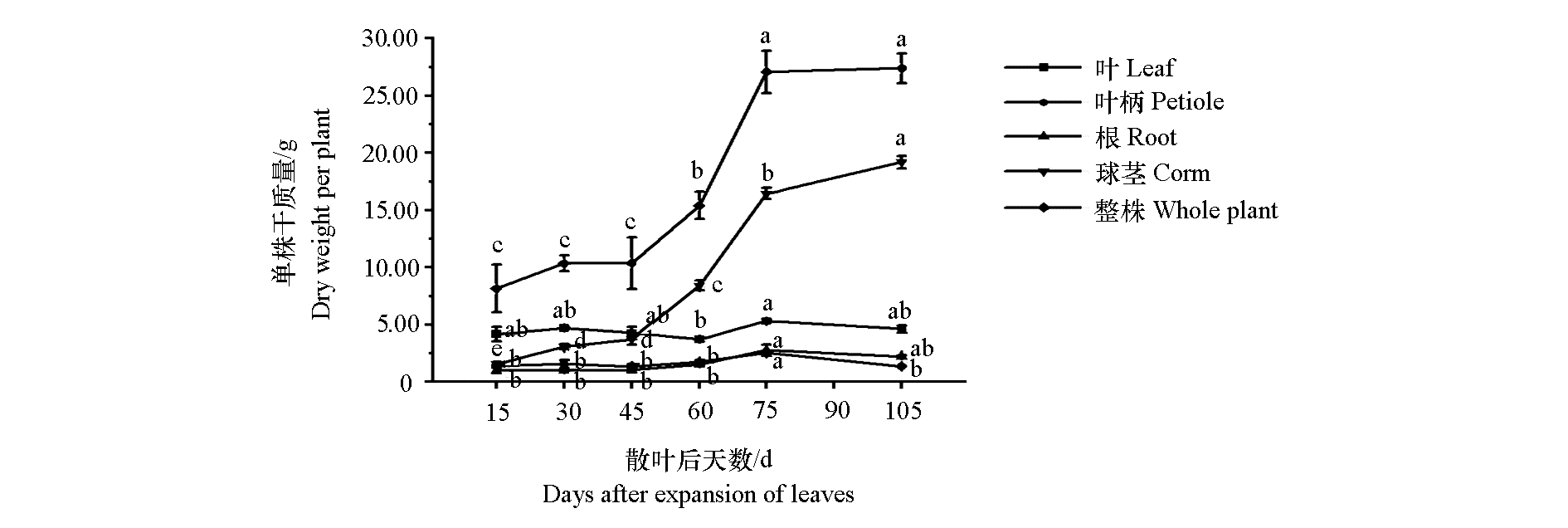
Fig. 1 The changes of dry weight of different organs during corm-forming stage of konjac LSD method was used for significance analysis;Different small letters represented significant differences at the level of P < 0.05. The same below.
| 散叶后天数/d Days after expansion of leaves | 叶片 Leaf | 叶柄 Petiole | 根 Root | 球茎 Corm |
|---|---|---|---|---|
| 30 | 35.33 ± 0.83 a | 15.58 ± 1.09 c | 29.00 ± 3.95 a | 12.21 ± 0.96 c |
| 45 | 34.90 ± 0.58 a | 19.02 ± 1.59 b | 28.84 ± 0.79 a | 21.06 ± 5.56 a |
| 60 | 35.68 ± 2.11 a | 21.79 ± 1.04 a | 29.36 ± 4.71 a | 17.25 ± 0.14 abc |
| 75 | 29.19 ± 0.71 b | 14.55 ± 1.15 c | 26.47 ± 2.44 a | 15.09 ± 2.19 bc |
| 105 | 23.84 ± 1.30 c | 15.69 ± 0.94 c | 23.81 ± 2.78 a | 17.95 ± 1.51 ab |
Table 2 The changes of nitrogen content in different organs during konjac corm-forming stage mg · g-1DW
| 散叶后天数/d Days after expansion of leaves | 叶片 Leaf | 叶柄 Petiole | 根 Root | 球茎 Corm |
|---|---|---|---|---|
| 30 | 35.33 ± 0.83 a | 15.58 ± 1.09 c | 29.00 ± 3.95 a | 12.21 ± 0.96 c |
| 45 | 34.90 ± 0.58 a | 19.02 ± 1.59 b | 28.84 ± 0.79 a | 21.06 ± 5.56 a |
| 60 | 35.68 ± 2.11 a | 21.79 ± 1.04 a | 29.36 ± 4.71 a | 17.25 ± 0.14 abc |
| 75 | 29.19 ± 0.71 b | 14.55 ± 1.15 c | 26.47 ± 2.44 a | 15.09 ± 2.19 bc |
| 105 | 23.84 ± 1.30 c | 15.69 ± 0.94 c | 23.81 ± 2.78 a | 17.95 ± 1.51 ab |
| 散叶后天数/d Days after expansion of leaves | 叶片 Leaf | 叶柄 Petiole | 根 Root | 球茎 Corm | 全株 Whole plant |
|---|---|---|---|---|---|
| 30 | 1.17 ± 0.04 a | 1.12 ± 0.10 ab | 1.12 ± 0.06 ab | 0.89 ± 0.11 b | 4.31 ± 0.19 a |
| 45 | 0.87 ± 0.22 b | 1.34 ± 0.17 a | 1.06 ± 0.16 b | 1.22 ± 0.04 a | 4.49 ± 0.58 a |
| 60 | 0.85 ± 0.12 b | 1.36 ± 0.16 a | 1.27 ± 0.09 a | 1.34 ± 0.08 a | 4.81 ± 0.44 a |
| 75 | 0.59 ± 0.11 c | 0.87 ± 0.17 b | 0.55 ± 0.02 d | 0.86 ± 0.12 b | 2.88 ± 0.38 b |
| 105 | 0.56 ± 0.04 c | 0.90 ± 0.05 b | 0.78 ± 0.06 c | 0.60 ± 0.02 c | 2.84 ± 0.07 b |
Table 3 Ndff values in different organs during corm-forming stage of konjac %
| 散叶后天数/d Days after expansion of leaves | 叶片 Leaf | 叶柄 Petiole | 根 Root | 球茎 Corm | 全株 Whole plant |
|---|---|---|---|---|---|
| 30 | 1.17 ± 0.04 a | 1.12 ± 0.10 ab | 1.12 ± 0.06 ab | 0.89 ± 0.11 b | 4.31 ± 0.19 a |
| 45 | 0.87 ± 0.22 b | 1.34 ± 0.17 a | 1.06 ± 0.16 b | 1.22 ± 0.04 a | 4.49 ± 0.58 a |
| 60 | 0.85 ± 0.12 b | 1.36 ± 0.16 a | 1.27 ± 0.09 a | 1.34 ± 0.08 a | 4.81 ± 0.44 a |
| 75 | 0.59 ± 0.11 c | 0.87 ± 0.17 b | 0.55 ± 0.02 d | 0.86 ± 0.12 b | 2.88 ± 0.38 b |
| 105 | 0.56 ± 0.04 c | 0.90 ± 0.05 b | 0.78 ± 0.06 c | 0.60 ± 0.02 c | 2.84 ± 0.07 b |
| 散叶后天数/d Days after expansion of leaves | 叶片 Leaf | 叶柄 Petiole | 根 Root | 球茎 Corm |
|---|---|---|---|---|
| 30 | 67.09 ± 0.38 a | 8.30 ± 0.24 b | 11.55 ± 1.11 bc | 13.06 ± 0.83 e |
| 45 | 45.98 ± 3.00 b | 11.99 ± 1.28 a | 12.68 ± 0.04 b | 29.34 ± 0.46 d |
| 60 | 26.62 ± 1.42 c | 13.03 ± 2.12 a | 14.97 ± 0.78 a | 45.38 ± 3.32 c |
| 75 | 24.04 ± 0.36 c | 8.53 ± 0.63 b | 10.77 ± 0.95 c | 56.66 ± 5.09 b |
| 105 | 19.12 ± 0.52 d | 5.51 ± 0.13 c | 12.13 ± 0.62 bc | 63.24 ± 4.62 a |
Table 4 The changes of 15N allocation rate in different organs during konjac corm-forming stage %
| 散叶后天数/d Days after expansion of leaves | 叶片 Leaf | 叶柄 Petiole | 根 Root | 球茎 Corm |
|---|---|---|---|---|
| 30 | 67.09 ± 0.38 a | 8.30 ± 0.24 b | 11.55 ± 1.11 bc | 13.06 ± 0.83 e |
| 45 | 45.98 ± 3.00 b | 11.99 ± 1.28 a | 12.68 ± 0.04 b | 29.34 ± 0.46 d |
| 60 | 26.62 ± 1.42 c | 13.03 ± 2.12 a | 14.97 ± 0.78 a | 45.38 ± 3.32 c |
| 75 | 24.04 ± 0.36 c | 8.53 ± 0.63 b | 10.77 ± 0.95 c | 56.66 ± 5.09 b |
| 105 | 19.12 ± 0.52 d | 5.51 ± 0.13 c | 12.13 ± 0.62 bc | 63.24 ± 4.62 a |
| 散叶后天数/d Days after expansion of leaves | 叶片 Leaf | 叶柄 Petiole | 根 Root | 球茎 Corm | 全株 Whole plant |
|---|---|---|---|---|---|
| 30 | 10.57 ± 0.06 a | 1.31 ± 0.04 bc | 1.82 ± 0.18 c | 2.06 ± 0.13 d | 15.75 ± 0.03 cd |
| 45 | 6.37 ± 0.42 b | 1.66 ± 0.18 b | 1.76 ± 0.01 c | 4.06 ± 0.07 c | 13.85 ± 0.66 d |
| 60 | 6.04 ± 0.33 b | 2.96 ± 0.48 a | 3.40 ± 0.18 a | 10.29 ± 0.75 b | 22.68 ± 1.73 a |
| 75 | 4.92 ± 0.07 c | 1.75 ± 0.13 b | 2.21 ± 0.20 b | 11.60 ± 1.04 a | 20.47 ± 1.44 b |
| 105 | 3.11 ± 0.08 d | 0.90 ± 0.02 c | 1.97 ± 0.10 bc | 10.29 ± 0.75 b | 16.27 ± 0.76 c |
Table 5 The changes of 15N utilization rate in different organs during konjac corm-forming stage %
| 散叶后天数/d Days after expansion of leaves | 叶片 Leaf | 叶柄 Petiole | 根 Root | 球茎 Corm | 全株 Whole plant |
|---|---|---|---|---|---|
| 30 | 10.57 ± 0.06 a | 1.31 ± 0.04 bc | 1.82 ± 0.18 c | 2.06 ± 0.13 d | 15.75 ± 0.03 cd |
| 45 | 6.37 ± 0.42 b | 1.66 ± 0.18 b | 1.76 ± 0.01 c | 4.06 ± 0.07 c | 13.85 ± 0.66 d |
| 60 | 6.04 ± 0.33 b | 2.96 ± 0.48 a | 3.40 ± 0.18 a | 10.29 ± 0.75 b | 22.68 ± 1.73 a |
| 75 | 4.92 ± 0.07 c | 1.75 ± 0.13 b | 2.21 ± 0.20 b | 11.60 ± 1.04 a | 20.47 ± 1.44 b |
| 105 | 3.11 ± 0.08 d | 0.90 ± 0.02 c | 1.97 ± 0.10 bc | 10.29 ± 0.75 b | 16.27 ± 0.76 c |
| 基因家族 Gene family | 基因名称 Gene name | 基因编号 Gene identifier | 等电点 pI | 分子量/ kD MW | 氨基酸数 Amino acid number | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|
| NR | AkNIA1 | evm.model.HIC_ASM_3.7576_Akon | 10.05 | 56.88 | 507 | 细胞质Cytoplasm |
| AkNIA2 | evm.model.HIC_ASM_3.7577_Akon | 8.85 | 53.58 | 486 | 细胞质Cytoplasm | |
| AkNIA3 | evm.model.CTG_10382.10_Akon | 8.91 | 14.69 | 131 | 细胞膜Cell membrane | |
| AkNIA4 | evm.model.CTG_15369.3_Akon | 7.87 | 13.07 | 118 | 细胞膜Cell membrane | |
| NIR | AkNIR1 | evm.model.HIC_ASM_7.230_Akon | 6.60 | 67.20 | 609 | 叶绿体,线粒体Chloroplast,mitochondrion |
| AkNIR2 | evm.model.CTG_4710.3_Akon | 6.60 | 67.20 | 609 | 叶绿体,线粒体Chloroplast,mitochondrion | |
| GS | AkGSR2-1 | evm.model.HIC_ASM_2.6744_Akon | 6.68 | 47.74 | 438 | 叶绿体,线粒体Chloroplast,mitochondrion |
| AkGSR2-2 | evm.model.HIC_ASM_2.6760_Akon | 6.68 | 47.81 | 438 | 叶绿体,线粒体Chloroplast,mitochondrion | |
| AkGSR2-3 | evm.model.HIC_ASM_3.9051_Akon | 6.22 | 39.42 | 358 | 叶绿体,细胞质Chloroplast,cytoplasm | |
| AkGSR2-4 | evm.model.HIC_ASM_5.508_Akon | 5.22 | 24.29 | 219 | 叶绿体,线粒体Chloroplast,mitochondrion | |
| AkGSR2-5 | evm.model.HIC_ASM_5.6034_Akon | 5.53 | 39.41 | 358 | 叶绿体Chloroplast | |
| AkGSR2-6 | evm.model.HIC_ASM_7.1708_Akon | 10.28 | 10.35 | 96 | 叶绿体Chloroplast | |
| AkGSR2-7 | evm.model.HIC_ASM_7.4324_Akon | 5.88 | 17.67 | 163 | 叶绿体Chloroplast | |
| AkGSR2-8 | evm.model.HIC_ASM_7.7618_Akon | 5.48 | 39.15 | 357 | 细胞质Cytoplasm | |
| AkGSR2-9 | evm.model.CTG_9235.4_Akon | 8.65 | 26.06 | 239 | 叶绿体Chloroplast | |
| AkGSR2-10 | evm.model.CTG_10208.4_evm.model.CTG_10208.5_Akon | 5.36 | 39.23 | 357 | 细胞质Cytoplasm | |
| AkGSR2-11 | evm.model.CTG_18461.3.3_Akon | 5.65 | 93.14 | 841 | 叶绿体Chloroplast | |
| GOGAT | AkGLU1 | evm.model.HIC_ASM_2.6868_Akon | 6.70 | 176.22 | 1 626 | 叶绿体Chloroplast |
| AkGLT1 | evm.model.HIC_ASM_8.1189_Akon | 6.47 | 242.63 | 2 209 | 叶绿体Chloroplast | |
| AS | AkASN1 | evm.model.HIC_ASM_4.2066_Akon | 6.09 | 70.65 | 634 | 叶绿体Chloroplast |
| AkASN2 | evm.model.HIC_ASM_4.950_Akon | 5.83 | 66.02 | 592 | 叶绿体,细胞质Chloroplast,cytoplasm |
Table 6 Characterization of nitrogen metabolism related gene families in Amorphophallus konjac
| 基因家族 Gene family | 基因名称 Gene name | 基因编号 Gene identifier | 等电点 pI | 分子量/ kD MW | 氨基酸数 Amino acid number | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|
| NR | AkNIA1 | evm.model.HIC_ASM_3.7576_Akon | 10.05 | 56.88 | 507 | 细胞质Cytoplasm |
| AkNIA2 | evm.model.HIC_ASM_3.7577_Akon | 8.85 | 53.58 | 486 | 细胞质Cytoplasm | |
| AkNIA3 | evm.model.CTG_10382.10_Akon | 8.91 | 14.69 | 131 | 细胞膜Cell membrane | |
| AkNIA4 | evm.model.CTG_15369.3_Akon | 7.87 | 13.07 | 118 | 细胞膜Cell membrane | |
| NIR | AkNIR1 | evm.model.HIC_ASM_7.230_Akon | 6.60 | 67.20 | 609 | 叶绿体,线粒体Chloroplast,mitochondrion |
| AkNIR2 | evm.model.CTG_4710.3_Akon | 6.60 | 67.20 | 609 | 叶绿体,线粒体Chloroplast,mitochondrion | |
| GS | AkGSR2-1 | evm.model.HIC_ASM_2.6744_Akon | 6.68 | 47.74 | 438 | 叶绿体,线粒体Chloroplast,mitochondrion |
| AkGSR2-2 | evm.model.HIC_ASM_2.6760_Akon | 6.68 | 47.81 | 438 | 叶绿体,线粒体Chloroplast,mitochondrion | |
| AkGSR2-3 | evm.model.HIC_ASM_3.9051_Akon | 6.22 | 39.42 | 358 | 叶绿体,细胞质Chloroplast,cytoplasm | |
| AkGSR2-4 | evm.model.HIC_ASM_5.508_Akon | 5.22 | 24.29 | 219 | 叶绿体,线粒体Chloroplast,mitochondrion | |
| AkGSR2-5 | evm.model.HIC_ASM_5.6034_Akon | 5.53 | 39.41 | 358 | 叶绿体Chloroplast | |
| AkGSR2-6 | evm.model.HIC_ASM_7.1708_Akon | 10.28 | 10.35 | 96 | 叶绿体Chloroplast | |
| AkGSR2-7 | evm.model.HIC_ASM_7.4324_Akon | 5.88 | 17.67 | 163 | 叶绿体Chloroplast | |
| AkGSR2-8 | evm.model.HIC_ASM_7.7618_Akon | 5.48 | 39.15 | 357 | 细胞质Cytoplasm | |
| AkGSR2-9 | evm.model.CTG_9235.4_Akon | 8.65 | 26.06 | 239 | 叶绿体Chloroplast | |
| AkGSR2-10 | evm.model.CTG_10208.4_evm.model.CTG_10208.5_Akon | 5.36 | 39.23 | 357 | 细胞质Cytoplasm | |
| AkGSR2-11 | evm.model.CTG_18461.3.3_Akon | 5.65 | 93.14 | 841 | 叶绿体Chloroplast | |
| GOGAT | AkGLU1 | evm.model.HIC_ASM_2.6868_Akon | 6.70 | 176.22 | 1 626 | 叶绿体Chloroplast |
| AkGLT1 | evm.model.HIC_ASM_8.1189_Akon | 6.47 | 242.63 | 2 209 | 叶绿体Chloroplast | |
| AS | AkASN1 | evm.model.HIC_ASM_4.2066_Akon | 6.09 | 70.65 | 634 | 叶绿体Chloroplast |
| AkASN2 | evm.model.HIC_ASM_4.950_Akon | 5.83 | 66.02 | 592 | 叶绿体,细胞质Chloroplast,cytoplasm |

Fig. 2 The analysis of nitrogen metabolism pathway and related gene tissue expression in konjac The gene transcription level is represented by FPKM values.
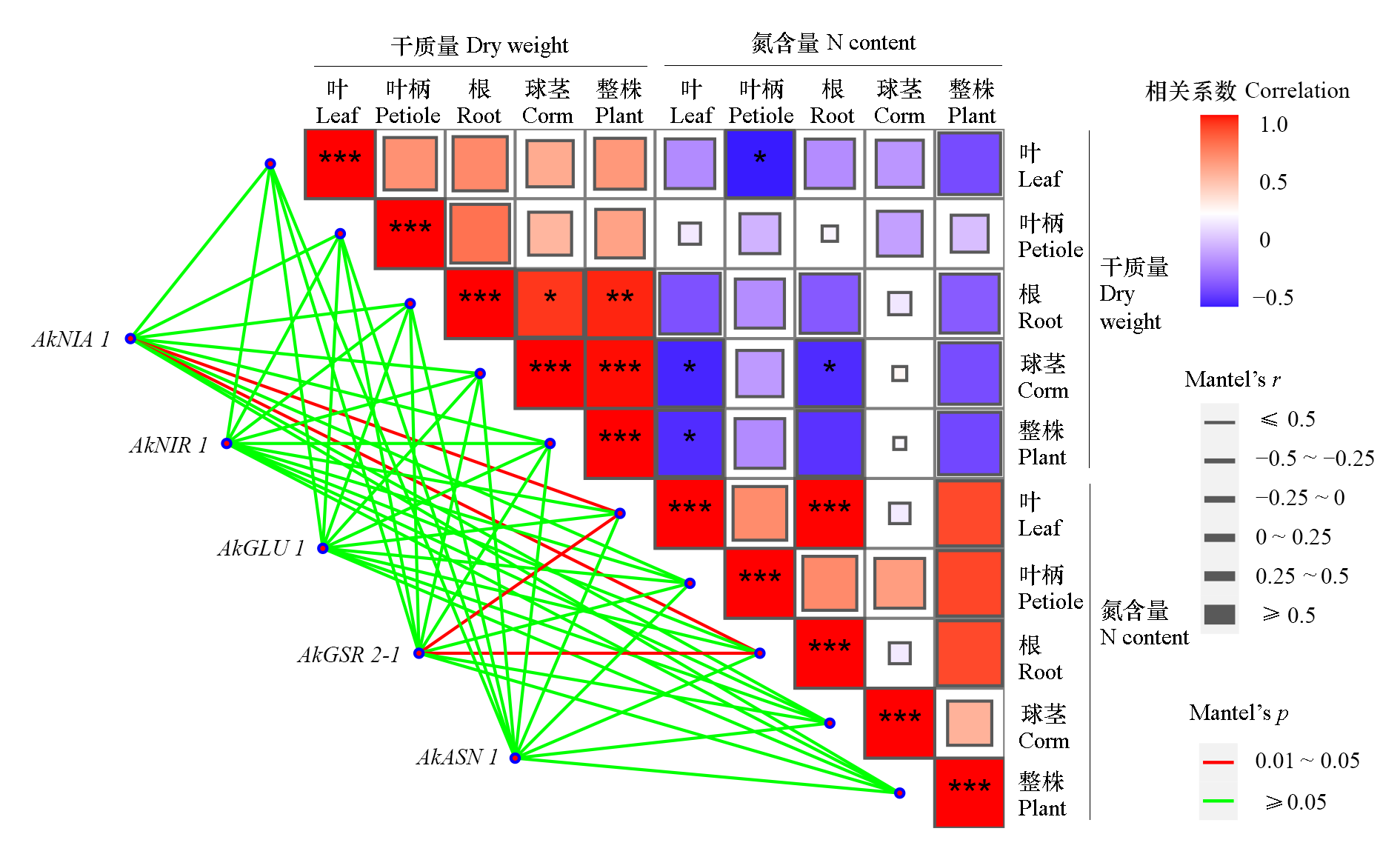
Fig. 4 The correlation analysis between dry weight, nitrogen content and expressions of key nitrogen metabolism-related genes in leaves during konjac corm-forming stage * P < 0.05,** P < 0.01,*** P < 0.001.
| [1] |
|
|
崔鸣, 赵兴喜, 都大俊, 刘列平, 王小兵, 李增义. 2006. 氮磷钾肥料对魔芋产量的影响效应研究. 水土保持研究,(6):185-187.
|
|
| [2] |
|
|
代雪凤, 朱丽, 张盛林, 牛义, 刘海利. 2021. 魔芋软腐病拮抗放线菌筛选. 西南大学学报(自然科学版), 43 (11):9-17.
|
|
| [3] |
|
|
丁永刚. 2022. 小麦高产高效品种特征及其氮响应机制[博士论文]. 扬州: 扬州大学.
|
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
|
黄建, 段婧婧, 马晓东, 祁通, 付彦博. 2020. 盐环境下氮素对苗期盐角草氮同化关键酶活性的影响. 新疆农业科学, 57 (7):1314-1320.
doi: 10.6048/j.issn.1001-4330.2020.07.016 |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
|
李成晨, 索海翠, 罗焕明, 安康, 刘计涛, 王丽, 单建伟, 杨少海, 李小波. 2021. 化肥减施和施肥方式对马铃薯产量和块茎氮素积累的影响. 中国农业科技导报, 23 (9):173-183.
|
|
| [12] |
|
|
李勇军, 马继琼, 陈建华, 尹桂芳, 方顺权, 徐云, 王玲. 2010. 施氮量对魔芋病害发生、产量及粘度影响的研究. 西南农业学报, 23 (1):128-131.
|
|
| [13] |
|
|
刘佩瑛. 2004. 魔芋学. 北京: 中国农业出版社.
|
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
|
门永阁, 安欣, 许海港, 姜翰, 魏绍冲, 姜远茂. 2015. 不同负载量对苹果13C和15N分配、利用的影响. 植物营养与肥料学报, 21 (3):702-708.
|
|
| [18] |
|
|
谭燕, 刘曦, 袁芳. 2019. 魔芋葡甘聚糖的结构、性质及其在食品中的应用. 中国调味品, 44 (2):168-174,178.
|
|
| [19] |
|
|
汤丹峰, 张新明, 陈洪, 全锋, 伍尤国, 曹先维. 2013. 冬种马铃薯的氮素吸收、积累、分配特征研究. 热带作物学报, 34 (6):1041-1044.
|
|
| [20] |
|
|
王前登, 陈波浪, 玉素甫江 · 玉素音, 王成, 柴仲平. 2018. 库尔勒香梨春季施用15N-尿素的吸收、分配和利用特性. 应用生态学报, 29 (5),1443-1449.
doi: 10.13287/j.1001-9332.201805.009 |
|
| [21] |
|
| [22] |
|
|
徐凯悦, 王晓华, 宋彪, 郭世伟, 郑朝元, 郭九信, 吴良泉, 苏达. 2021. 不同时期追施氮肥对成熟期蜜柚树体氮素分配的影响. 植物营养与肥料学报, 27 (4):553-564.
|
|
| [23] |
|
|
信珊珊. 2019. 魔芋葡甘聚糖的生理功效综述. 粮食与食品工业, 26 (5):50-52.
|
|
| [24] |
|
| [25] |
|
| [26] |
|
|
杨秀莲, 寸湘琴, 梁艳丽, 谢世清, 赵庆云. 2014. 不同氮、磷、钾肥施用量对魔芋软腐病的影响. 长江蔬菜,(22):69-71.
|
|
| [27] |
|
|
袁昊田, 蒙美莲, 陈有君, 谭伟林, 王占忠. 2020. 马铃薯不同品种氮素吸收转运规律. 中国马铃薯, 34 (1):22-30.
|
|
| [28] |
|
|
赵大伟, 刘炜林, 青方具, 陈瑞, 徐杰, 孟凡来, 杨童舒. 2021. 花魔芋间作糯玉米的高产高效模式分析. 热带农业科学, 41 (1):24-30.
|
|
| [29] |
|
| [30] |
|
| [31] |
|
|
张婷婷, 孟丽丽, 刘晓蕊, 陈有君, 刘坤雨, 袁昊田, 蒙美莲. 2022. 马铃薯氮代谢对低氮胁迫的响应及转录组分析. 西北农林科技大学学报(自然科学版), 50 (8):15-26.
|
|
| [32] |
|
|
张忠学, 陈鹏, 陈帅宏, 郑恩楠, 聂堂哲, 刘明. 2018. 15N示踪分析节水灌溉下水稻对不同时期氮肥的吸收分配. 农业机械学报, 49 (6),309-317,346.
|
| [1] | ZHOU Ping, YAN Shaobin, GUO Rui, JIN Guang. Identification and Expressional Analysis of MGT Gene Family in Prunus persica [J]. Acta Horticulturae Sinica, 2024, 51(3): 463-478. |
| [2] | CUI Yiqiong, LI Ju, LIU Xiaoqi, WANG Junwen, TANG Zhongqi, WU Yue, XIAO Xuemei, YU Jihua. Effects of Water Deficiency on Sucrose and Starch Metabolism of Substrate Culture Tomato in Greenhouse [J]. Acta Horticulturae Sinica, 2024, 51(11): 2607-2619. |
| [3] | HAN Shiwen, LIU Tao, WANG Liping, LI Nanyang, WANG Suna, WANG Xing. Genome-Wide Identification and Stress-Responsive Expression Analysis of the Cucumber SRS Gene Family [J]. Acta Horticulturae Sinica, 2024, 51(10): 2281-2296. |
| [4] | YOU Qian, LIU Xiao, LIU Mengmeng, LIU Dan, BO Chen, ZHU Yanfang, DUAN Yongbo, XUE Jianping, ZHANG Aimin, XUE Tao. Identification and Bioinformatics Analysis of the HSF Family Gene in Pinellia ternata [J]. Acta Horticulturae Sinica, 2024, 51(10): 2371-2385. |
| [5] | YE Yufan, WANG Yujie, FU Qianyuan, WANG Lu, HAO Xinyuan, DING Changqing, WANG Xinchao, CAO Hongli, LI Nana. Cloning and Expression Analysis of Mg-Chelatase H Subunit Gene CsChlH in Tea Plant(Camellia sinensis) [J]. Acta Horticulturae Sinica, 2024, 51(1): 91-102. |
| [6] | WANG Xiaochen, NIE Ziye, LIU Xianju, DUAN Wei, FAN Peige, LIANG Zhenchang. Effects of Abscisic Acid on Monoterpene Synthesis in‘Jingxiangyu’Grape Berries [J]. Acta Horticulturae Sinica, 2023, 50(2): 237-249. |
| [7] | SHEN Yuxiao, ZOU Jinyu, LUO Ping, SHANG Wenqian, LI Yonghua, HE Songlin, WANG Zheng, SHI Liyun. Genome-wide Identification and Abiotic Stress Response Analysis of PP2C Family Genes in Rosa chinensis‘Old Blush’ [J]. Acta Horticulturae Sinica, 2023, 50(10): 2139-2156. |
| [8] | ZHAI Hanhan, ZHAI Yujie, TIAN Yi, ZHANG Ye, YANG Li, WEN Zhiliang, CHEN Haijiang. Genome-wide Identification of Peach SAUR Gene Family and Characterization of PpSAUR5 Gene [J]. Acta Horticulturae Sinica, 2023, 50(1): 1-14. |
| [9] | ZHANG Qiuyue, LIU Changlai, YU Xiaojing, YANG Jiading, FENG Chaonian. Screening of Reference Genes for Differentially Expressed Genes in Pyrus betulaefolia Plant Under Salt Stress by qRT-PCR [J]. Acta Horticulturae Sinica, 2022, 49(7): 1557-1570. |
| [10] | LIU Shangjia, L& Yao, CAO Bili, CHEN Zijing, GAO Song, XU Kun. Effects of High Temperature and Waterlogging Stress on Photosynthesis and Nitrogen Metabolism of Ginger Leaves [J]. Acta Horticulturae Sinica, 2022, 49(5): 1073-1080. |
| [11] | LI Yamei, MA Fuli, ZHANG Shanqi, HUANG Jinqiu, CHEN Mengting, ZHOU Junyong, SUN Qibao, SUN Jun. Optimization of Jujube Callus Transformation System and Application of ZjBRC1 in Regulating ZjYUCCA Expression [J]. Acta Horticulturae Sinica, 2022, 49(4): 749-757. |
| [12] | WANG Ying, AI Penghui, LI Shuailei, KANG Dongru, LI Zhongai, WANG Zicheng. Identification and Expression Analysis of Genes Related to DNA Methylation in Chrysanthemum × morifolium and C. nankingense [J]. Acta Horticulturae Sinica, 2022, 49(4): 827-840. |
| [13] | ZHANG Rui, ZHANG Xiayi, ZHAO Ting, WANG Shuangcheng, ZHANG Zhongxing, LIU Bo, ZHANG De, WANG Yanxiu. Transcriptome Analysis of the Molecular Mechanism of Saline-alkali Stress Response in Malus halliana Leaves [J]. Acta Horticulturae Sinica, 2022, 49(2): 237-251. |
| [14] | ZHOU Zhiming, YANG Jiabao, ZHANG Cheng, ZENG Linglu, MENG Wanqiu, SUN Li. Genome-wide Identification and Expression Analyses of Long-chain Acyl-CoA Synthetases Under Abiotic Stresses in Helianthus annuus [J]. Acta Horticulturae Sinica, 2022, 49(2): 352-364. |
| [15] | QIAO Jun, WANG Liying, LIU Jing, LI Suweng. Expression Analysis of Genes Related to Photosensitive Color Under the Caylx in Eggplant Based on Transcriptome Sequencing [J]. Acta Horticulturae Sinica, 2022, 49(11): 2347-2356. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2012 Acta Horticulturae Sinica 京ICP备10030308号-2 国际联网备案号 11010802023439
Tel: 010-82109523 E-Mail: yuanyixuebao@126.com
Support by: Beijing Magtech Co.Ltd