
Acta Horticulturae Sinica ›› 2021, Vol. 48 ›› Issue (8): 1565-1578.doi: 10.16420/j.issn.0513-353x.2020-0660
• Research Papers • Previous Articles Next Articles
YANG Tianchen, CHEN Xiaotong, LÜ Ke, ZHANG Di( )
)
Received:2021-02-09
Revised:2021-06-03
Online:2021-08-25
Published:2021-09-06
Contact:
ZHANG Di
E-mail:zhangdi2013@sjtu.edu.cn
CLC Number:
YANG Tianchen, CHEN Xiaotong, LÜ Ke, ZHANG Di. Expression Pattern and Regulation Mechanism of ApSK3 Dehydrin (Agapanthus praecox)Response to Abiotic Stress and Hormone Signals[J]. Acta Horticulturae Sinica, 2021, 48(8): 1565-1578.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.ahs.ac.cn/EN/10.16420/j.issn.0513-353x.2020-0660
| 引物名称 | 序列(5′-3′) | 退火温度/℃ |
|---|---|---|
| Primer name | Primer sequence | Tm |
| Ap-Actin-S | CAGTGTCTGGATTGGAGG | 50.0 |
| Ap-Actin-A | TAGAAGCACTTCCTGTG | 50.0 |
| RT-ApSK3-S | AAGAGCCAAGAGGAGGTT | 55.0 |
| RT-ApSK3-A | CTTCTTCTCGCCGTCTTC | 55.0 |
| ApSK3-SP1 | TGTGGCCGGGGAGCTTCTGTTT | 61.4 |
| ApSK3-SP2 | ACGTCCTGTTCGGTTACCACGG | 61.4 |
| ApSK3-SP3 | CCACTTCCTCTTCGTCGCTCGA | 61.4 |
| ApSK3-SP2-1 | AGCTAGAGAAACGAATTATCACATCCCTC | 59.6 |
| ApSK3-SP2-2 | ACTTTCAAGCTCTCGACTCCGG | 59.5 |
| ApSK3-SP2-3 | CCCTCTCTCACTCACCTCCACA | 61.4 |
| Sp-2175-S | ATGACCATGATTACGCCAAGCTTGTGTTGCTATTTGTTAGAGAA | 56.0 |
| Sp-1167-S | ATGACCATGATTACGCCAAGCTTAAAGTAAAAAGAGCCAACACTTG | 55.0 |
| Sp-950-S | ATGACCATGATTACGCCAAGCTTCCACATCCGTCCATTAGTGC | 57.0 |
| Sp-646-S | ATGACCATGATTACGCCAAGCTTGGCAACATAATCATTAAGATACTGT | 57.0 |
| Sp-291-S | ATGACCATGATTACGCCAAGCTTGCTGATGGATAAGTAAAGAATAA | 55.0 |
| Sp-A | ACTGACCACCCGGGGATCCTTTTTTAATTAATTATAAACTTCAATG | 59.0 |
| pBI121-S | CGGCTCGTATGTTGTGTGGAATTG | 60.0 |
| pBI121-A | CGTTGGGGTTTCTACAGGACGTAA | 58.0 |
| Kana-S | TGGATTGCACGCAGGTTCTC | 58.0 |
| Kana-A | CTCGATGCGATGTTTCGCTT | 58.0 |
Table 1 Corresponding primer sequences
| 引物名称 | 序列(5′-3′) | 退火温度/℃ |
|---|---|---|
| Primer name | Primer sequence | Tm |
| Ap-Actin-S | CAGTGTCTGGATTGGAGG | 50.0 |
| Ap-Actin-A | TAGAAGCACTTCCTGTG | 50.0 |
| RT-ApSK3-S | AAGAGCCAAGAGGAGGTT | 55.0 |
| RT-ApSK3-A | CTTCTTCTCGCCGTCTTC | 55.0 |
| ApSK3-SP1 | TGTGGCCGGGGAGCTTCTGTTT | 61.4 |
| ApSK3-SP2 | ACGTCCTGTTCGGTTACCACGG | 61.4 |
| ApSK3-SP3 | CCACTTCCTCTTCGTCGCTCGA | 61.4 |
| ApSK3-SP2-1 | AGCTAGAGAAACGAATTATCACATCCCTC | 59.6 |
| ApSK3-SP2-2 | ACTTTCAAGCTCTCGACTCCGG | 59.5 |
| ApSK3-SP2-3 | CCCTCTCTCACTCACCTCCACA | 61.4 |
| Sp-2175-S | ATGACCATGATTACGCCAAGCTTGTGTTGCTATTTGTTAGAGAA | 56.0 |
| Sp-1167-S | ATGACCATGATTACGCCAAGCTTAAAGTAAAAAGAGCCAACACTTG | 55.0 |
| Sp-950-S | ATGACCATGATTACGCCAAGCTTCCACATCCGTCCATTAGTGC | 57.0 |
| Sp-646-S | ATGACCATGATTACGCCAAGCTTGGCAACATAATCATTAAGATACTGT | 57.0 |
| Sp-291-S | ATGACCATGATTACGCCAAGCTTGCTGATGGATAAGTAAAGAATAA | 55.0 |
| Sp-A | ACTGACCACCCGGGGATCCTTTTTTAATTAATTATAAACTTCAATG | 59.0 |
| pBI121-S | CGGCTCGTATGTTGTGTGGAATTG | 60.0 |
| pBI121-A | CGTTGGGGTTTCTACAGGACGTAA | 58.0 |
| Kana-S | TGGATTGCACGCAGGTTCTC | 58.0 |
| Kana-A | CTCGATGCGATGTTTCGCTT | 58.0 |

Fig. 1 DNA sequence analysis of ApSK3 promoter and the description of cis-elements The“A”of the translation initiation code“ATG”of ApSK3 is designated as“+ 1”. These important cis-elements related with stress and hormone are red markerd. The horizontal arrows show their directions. The vertical arrows above the sequence indicate the start point of different deletion fragments;the blue nucleotide sequences represent special primers for amplifying deletion fragments(Sp-S).
| 类别 | 名称 | 描述 | 核心序列 | 数量 | 位置/bp |
|---|---|---|---|---|---|
| Category | Name | Description | Core Sequence | Number | Position |
| 结构元件 Structure elements | TATA-Box | 转录起始-30核心启动子元件 Core promoter element around-30 of transcription start | TATATA (5) | 16 | 123 ~-128,-1314 ~-1319, -1704 ~-1709, -1928 ~-1933, -1973 ~-1978 |
| TATAAA (1) | -595 ~-600 | ||||
| TATA (7) | 36 ~-39,-192~-195,-478 ~-481,-507 ~-510,-1208 ~-1211,-1753 ~-1756, -1778 ~-1781 | ||||
| TTTATA (3) | -15 ~-20,-1385 ~ -1390,-1459 ~-1464 | ||||
| CAAT-Box | 启动子和增强子区域调控元件 Common cis-acting element in promoter and enhancer regions | CAAT (9) | 15 | -89 ~-92,-822 ~-825, -991 ~-994,-1002 ~ -1005,-1661 ~-1664, -1713 ~-1716,-1840 ~ -1843,-1965 ~-1968, -2010 ~-2013 | |
| CAAAT (6) | -340 ~-344,-492 ~ -496,-1359 ~-1363, -1367 ~-1370,-1558 ~ -1561,-2068 ~-2072 | ||||
| 胁迫响应元件Abiotic stress responsive elements | ARE | 厌氧顺式调控元件 cis-Acting regulatory element essential for the anaerobic induction | AACCA | 2 | -584 ~-588, -1534 ~-1538 |
| MBS | 干旱诱导MYB结合位点 MYB binding site involved in drought-inducibility | CAACTG CAACAG | 2 | -1512 ~-1517, -319 ~-324 | |
| circadian | 昼夜顺式调控元件 Circadian-control element | CAAAGTTATC | 1 | -1628 ~-1637 | |
| TC-rich repeats | 防卫和胁迫响应顺式调控元件 cis-Acting element involved in defense and stress responsiveness | GTTTTCTAA ATTCTCTAAC | 2 | -1127 ~-1135 -2159 ~-2168 | |
| 激素响应元件Phytohor- mone responsive element | ABRE | 脱落酸响应顺式调控元件 Abscisic acid responsive element | CACGTG | 2 | -977 ~-982, -881 ~-886 |
| CGTCA-motif | 茉莉酸甲酯响应顺式调控元件 MeJA-responsive element | CGTCA | 2 | -165 ~-169, -1370 ~-1374 | |
| ERE | 乙烯响应顺式调控元件 Ethylene-responsive element | ATTTCAAA | 1 | -526 ~-533 | |
| P-box | 赤霉素响应顺式调控元件 Gibberellin-responsive element | CCTTTTG | 1 | -561 ~-567 | |
| TGA-box | 生长素响应顺式调控元件 Auxin-responsive element | CGGTGCAGT | 1 | -170 ~-178 | |
| 生长发育调控元件 Development-related elements | Skn-1 motif | 胚乳表达调控顺式作用元件 cis-Acting element involved in endosperm expression | GTCAT | 5 | -164 ~-168,-1005 ~-1009,-1785 ~-1789, -1956 ~-1960, -2023 ~-2027 |
| GCN4 motif | 胚乳表达调控顺式作用元件 cis-Acting element involved in endosperm expression | CAAGCCA | 1 | -1650 ~-1656 |
Table 2 Identification of cis-acting elements and functions in the ApSK3 promoter
| 类别 | 名称 | 描述 | 核心序列 | 数量 | 位置/bp |
|---|---|---|---|---|---|
| Category | Name | Description | Core Sequence | Number | Position |
| 结构元件 Structure elements | TATA-Box | 转录起始-30核心启动子元件 Core promoter element around-30 of transcription start | TATATA (5) | 16 | 123 ~-128,-1314 ~-1319, -1704 ~-1709, -1928 ~-1933, -1973 ~-1978 |
| TATAAA (1) | -595 ~-600 | ||||
| TATA (7) | 36 ~-39,-192~-195,-478 ~-481,-507 ~-510,-1208 ~-1211,-1753 ~-1756, -1778 ~-1781 | ||||
| TTTATA (3) | -15 ~-20,-1385 ~ -1390,-1459 ~-1464 | ||||
| CAAT-Box | 启动子和增强子区域调控元件 Common cis-acting element in promoter and enhancer regions | CAAT (9) | 15 | -89 ~-92,-822 ~-825, -991 ~-994,-1002 ~ -1005,-1661 ~-1664, -1713 ~-1716,-1840 ~ -1843,-1965 ~-1968, -2010 ~-2013 | |
| CAAAT (6) | -340 ~-344,-492 ~ -496,-1359 ~-1363, -1367 ~-1370,-1558 ~ -1561,-2068 ~-2072 | ||||
| 胁迫响应元件Abiotic stress responsive elements | ARE | 厌氧顺式调控元件 cis-Acting regulatory element essential for the anaerobic induction | AACCA | 2 | -584 ~-588, -1534 ~-1538 |
| MBS | 干旱诱导MYB结合位点 MYB binding site involved in drought-inducibility | CAACTG CAACAG | 2 | -1512 ~-1517, -319 ~-324 | |
| circadian | 昼夜顺式调控元件 Circadian-control element | CAAAGTTATC | 1 | -1628 ~-1637 | |
| TC-rich repeats | 防卫和胁迫响应顺式调控元件 cis-Acting element involved in defense and stress responsiveness | GTTTTCTAA ATTCTCTAAC | 2 | -1127 ~-1135 -2159 ~-2168 | |
| 激素响应元件Phytohor- mone responsive element | ABRE | 脱落酸响应顺式调控元件 Abscisic acid responsive element | CACGTG | 2 | -977 ~-982, -881 ~-886 |
| CGTCA-motif | 茉莉酸甲酯响应顺式调控元件 MeJA-responsive element | CGTCA | 2 | -165 ~-169, -1370 ~-1374 | |
| ERE | 乙烯响应顺式调控元件 Ethylene-responsive element | ATTTCAAA | 1 | -526 ~-533 | |
| P-box | 赤霉素响应顺式调控元件 Gibberellin-responsive element | CCTTTTG | 1 | -561 ~-567 | |
| TGA-box | 生长素响应顺式调控元件 Auxin-responsive element | CGGTGCAGT | 1 | -170 ~-178 | |
| 生长发育调控元件 Development-related elements | Skn-1 motif | 胚乳表达调控顺式作用元件 cis-Acting element involved in endosperm expression | GTCAT | 5 | -164 ~-168,-1005 ~-1009,-1785 ~-1789, -1956 ~-1960, -2023 ~-2027 |
| GCN4 motif | 胚乳表达调控顺式作用元件 cis-Acting element involved in endosperm expression | CAAGCCA | 1 | -1650 ~-1656 |

Fig. 2 Expression level of ApSK3 in different tissues of Agapanthus praecox The dotted line indicated the expression level of Ap-actin(internal reference gene).
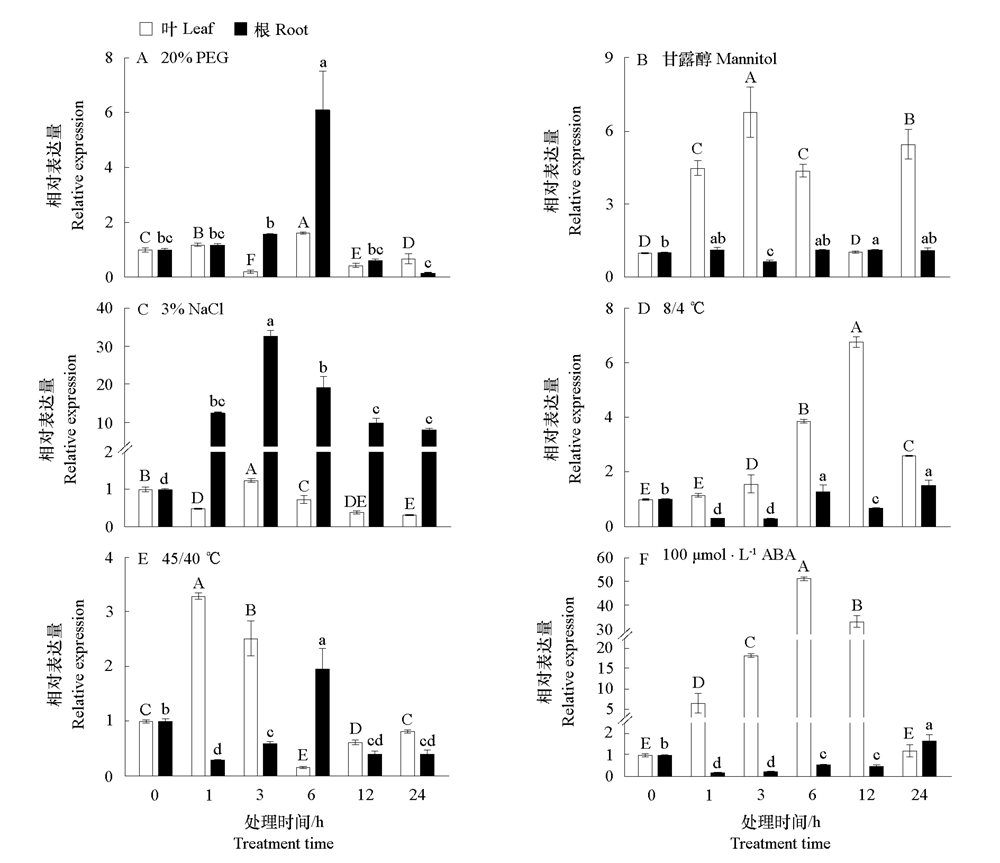
Fig. 4 Quantitative real-time PCR analysis of ApSK3 gene transcripts in Agapanthus praecox roots and leaves in response to various abiotic stresses and ABA treatment Ap-actin was used as internal reference gene. Values with different uppercase and lowercase letters are significantly different among samples in the roots and leaves,respectively.(P < 0.05).
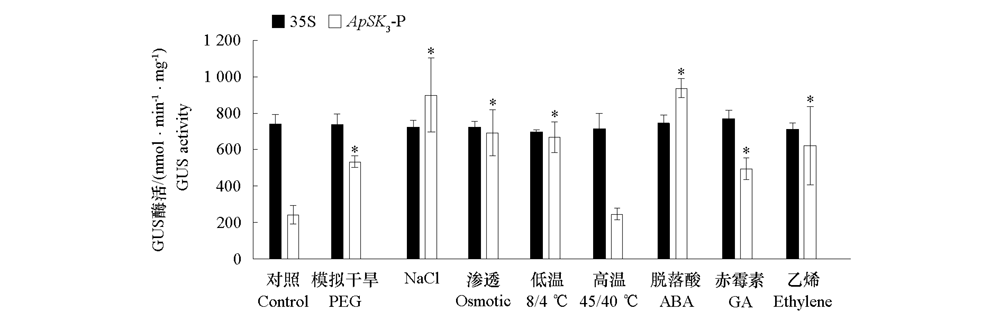
Fig. 5 GUS activity analysis of transgenic Arabidopsis seedlings containing ApSK3-P or 35S promoter under abiotic,ABA,GA and Ethylene treatments Asterisks(*)indicate significant differences between ApSK3-P under different treatments and control.(P < 0.05,α = 0.05). Ap-actin was used as internal reference gene. Values with different uppercase and lowercase letters are significantly different among samples in the roots and leaves,respectively.
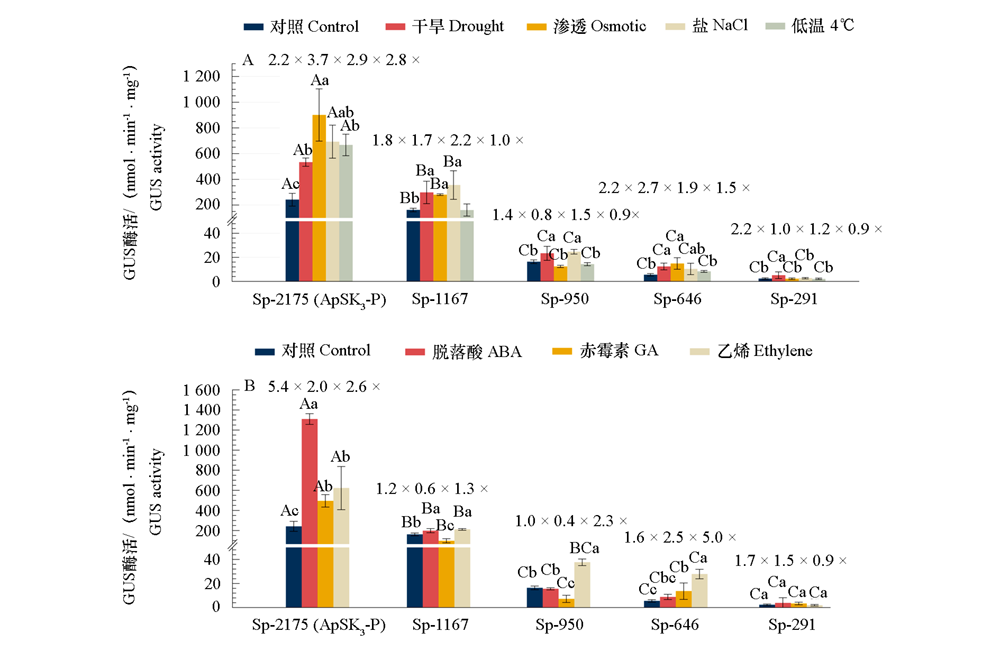
Fig. 7 GUS activity of transgenic Arabidopsis seedlings containing ApSK3 promoter and its 5' deletion constructs under abiotic(A)and ABA,GA,Ethylene(B)treatments Capital letters represent the significance of difference between the differernt ApSK3 promoters in the same treated condition;lower-case letters represent the significance among the same ApSK3 promoter under different treatments(P < 0.05,least significant difference test);numbers represent the fold of the same ApSK3 promoter under different treatments/Control.
| [1] |
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2003. Arabidopsis AtMYC2(bHLH)and AtMYB2(MYB)function as transcriptional activators in abscisic acid signaling. The Plant Cell, 15 (1):63-78.
doi: 10.1105/tpc.006130 URL |
| [2] |
Abedini R, GhaneGolmohammadi F, PishkamRad R, Pourabed E, Jafarnezhad A, Shobbar Z S, Shahbazi M. 2017. Plant dehydrins:shedding light on structure and expression patterns of dehydrin gene family in barley. Journal of Plant Research, 130 (4):747-763.
doi: 10.1007/s10265-017-0941-5 pmid: 28389925 |
| [3] | Allagulova C R, Gimalov F R, Shakirova F M, Vakhitov V A. 2003. The plant dehydrins:structure and putative functions. Biochemistry(Moscow), 68 (9):945-51. |
| [4] |
Bradford M M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72 (s 1-2):248-254.
doi: 10.1016/0003-2697(76)90527-3 URL |
| [5] |
Chen R G, Jing H, Guo W L, Wang S B, Ma F, Pan B G, Gong Z H. 2015. Silencing of dehydrin CaDHN1 diminishes tolerance to multiple abiotic stresses in Capsicum annuum L. Plant Cell Reports, 34 (12):2189-2200.
doi: 10.1007/s00299-015-1862-1 URL |
| [6] | Guo X Y, Liu D F, Chong K. 2018. Cold signaling in plants:insights into mechanisms and regulation. Journal of Integrative Plant Biology, 60 (9):7-18. |
| [7] |
Hernandez-Garcia C M, Finer J J. 2014. Identification and validation of promoters and cis-acting regulatory elements. Plant Science, 217-218 (1):109-119.
doi: 10.1016/j.plantsci.2013.12.007 URL |
| [8] |
Hobo T, Asada M, Kowyama Y, Hattori T. 1999. ACGT‐containing abscisic acid response element(ABRE)and coupling element 3(CE3)are functionally equivalent. The Plant Journal, 19 (6):679-689.
doi: 10.1046/j.1365-313x.1999.00565.x URL |
| [9] |
Hundertmark M, Hincha D K. 2008. LEA(Late Embryogenesis Abundant)proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics, 9 (1):118.
doi: 10.1186/1471-2164-9-118 URL |
| [10] |
Hwang S H, Lee I A, Yie S W, Hwang D J. 2008. Identification of an OsPR10a promoter region responsive to salicylic acid. Planta, 227 (5):1141-1150.
doi: 10.1007/s00425-007-0687-8 URL |
| [11] |
Jia F J, Qi S D, Li H, Liu P, Li P C, Wu C G, Zheng C C, Huang J G. 2014. Overexpression of Late Embryogenesis Abundant 14enhances Arabidopsis salt stress tolerance. Biochemical and Biophysical Research Communications, 454 (4):505-511.
doi: 10.1016/j.bbrc.2014.10.136 URL |
| [12] |
Kidokoro S, Watanabe K, Ohori T, Moriwaki T, Maruyama K, Mizoi J, Htwe N, Fujita Y, Sekita S, Shinozaki K, Yamaguchi-Shinozaki K. 2015. Soybean DREB1/CBF‐type transcription factors function in heat and drought as well as cold stress‐responsive gene expression. The Plant Journal, 81 (3):505-518.
doi: 10.1111/tpj.12746 pmid: 25495120 |
| [13] |
Kruger C, Berkowitz O, Stephan U W, Hell R. 2002. A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. Journal of Biological Chemistry, 277 (28):25062-25069.
doi: 10.1074/jbc.M201896200 URL |
| [14] |
Liang D, Xia H, Wu S, Ma F W. 2012. Genome-wide identification and expression profiling of dehydrin gene family in Malus domestica. Molecular Biology Reports, 39 (12):10759-10768.
doi: 10.1007/s11033-012-1968-2 URL pmid: 23053973 |
| [15] |
Liu H, Yu C Y, Li H X, Ouyang B, Wang T T, Zhang J H, Wang X, Ye Z B. 2015. Overexpression of ShDHN,a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Science, 231:198-211.
doi: 10.1016/j.plantsci.2014.12.006 URL |
| [16] | Liu Yang. 2014. Isolation and functional analysis of LEA protein genes, ZmDHN13,ZmLEA3 and ZmLEA5C in Zea mays[Ph. D. Dissertation]. Tai’an:Shandong Agricultural University. (in Chinese) |
| 刘洋. 2014. 玉米LEA蛋白基因ZmDHN13、ZmLEA3和ZmLEA5C的分离与功能分析[博士论文]. 泰安: 山东农业大学. | |
| [17] |
Lü A M, Fan N N, Xie J P, Yuan S L, An Y, Zhou P. 2017. Expression of CdDHN4,a novel YSK2-type dehydrin gene from bermudagrass,responses to drought stress through the ABA-dependent signal pathway. Frontiers in Plant Science, 8:748.
doi: 10.3389/fpls.2017.00748 URL |
| [18] | Ma Jie, Liu Cui-fang, Li Ling-zhi, Xiang Jian-hua, Chen Xin-bo. 2012. Progress in research of dehydrin response to abiotic stress. Journal of Biology, 29 (1):71-74. (in Chinese) |
| 马杰, 刘翠芳, 李灵之, 向建华, 陈信波. 2012. 非生物胁迫下植物脱水素的研究进展. 生物学杂志, 29 (1):71-74. | |
| [19] |
Nakashima K, Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K. 1997. A nuclear gene,erd1,encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. The Plant Journal, 12 (4):851-861.
doi: 10.1046/j.1365-313X.1997.12040851.x URL |
| [20] |
Pellegrineschi A, Reynolds M, Pacheco M, Brito R M, Almeraya R, Yamaguchi-Shinozaki K, Hoisington D. 2004. Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome, 47 (3):493-500.
pmid: 15190366 |
| [21] | Qian Gang. 2007. Genotypic variability in sequences and expression of LEA2/LEA3 genes in Tibetan Hulless Barley,Hordeum vulgare ssp. vulgare,associated with resistance to water deficit[Ph. D. Dissertation]. Chengdu:Chengdu Institute of Biology,the Chinese Academy of Sciences. (in Chinese) |
| 钱刚. 2007. 不同抗旱性青稞LEA2/LEA3蛋白基因的克隆与表达[博士论文]. 成都: 中国科学院研究生院. | |
| [22] |
Robertson M, Cuming A C, Chandler P M. 1995. Sequence analysis and hormonal regulation of a dehydrin promoter from barley, Hordeum vulgare. Physiologia Plantarum, 94 (3):470-478.
doi: 10.1111/ppl.1995.94.issue-3 URL |
| [23] | Rorat T. 2007. Plant dehydrins——Tissue location,structure and function. Cellular & Molecular Biology Letters, 11 (4):536-556. |
| [24] |
Rorat T, Grygorowicz W J, Irzykowski W, Rey P. 2004. Expression of KS-type dehydrins is primarily regulated by factors related to organ type and leaf developmental stage during vegetative growth. Planta, 218 (5):878-885.
URL pmid: 14685858 |
| [25] |
Saibi W, Feki K, Ben Mahmoud R, Brini F. 2015. Durum wheat dehydrin(DHN-5)confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta, 242 (5):1187-1194.
doi: 10.1007/s00425-015-2351-z URL |
| [26] | Swarup R, Parry G, Graham N, Allen T, Bennett M. 2002. Auxin cross-talk:integration of signalling pathways to control plant development. Plant Molecular Biology, 49 (3-4):411-426. |
| [27] | Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. 2000. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proceedings of the National Academy of Sciences of the United States of America, 97 (21):11632-11637. |
| [28] |
Vasil V, Marcotte W R,Jr, Rosenkrans L, Cocciolone S M, Vasil I K, Quatrano R S, McCarty D R. 1995. Overlap of Viviparous1( VP1)and abscisic acid response elements in the Em promoter:G-box elements are sufficient but not necessary for VP1 transactivation. Plant Cell, 7:1511-1518.
pmid: 8589631 |
| [29] |
Wang C H, Gao G, Cao S X, Xie Q J, Qi H Y. 2019. Isolation and functional validation of the CmLOX08 promoter associated with signalling molecule and abiotic stress responses in oriental melon,Cucumis melo var. makuwa Makino. BMC Plant Biology, 19 (1):75.
doi: 10.1186/s12870-019-1678-1 URL |
| [30] |
Wang H T, Zhang Y M, Xiao N, Zhang G, Wang F, Chen X Y, Fang R X. 2020a. Rice GERMIN-LIKE PROTEIN 2-1 functions in seed dormancy under the control of abscisic acid and gibberellic acid signaling pathways. Plant Physiology, 183 (3):1157-1170.
doi: 10.1104/pp.20.00253 URL |
| [31] | Wang Y D, Chen G J, Lei J J, Cao B H, Chen C M. 2020b. Identification and characterization of a LEA-like Gene,CaMF5,specifically expressed in the anthers of male-fertile Capsicum annuum. Horticultural Plant Journal, 26 (1):39-48. |
| [32] | Wu Qiong. 2019. Study of ABA-ethylene interaction on the regulation of cherry tomato fruit ripening[Ph. D. Dissertation]. Hangzhou:Zhejiang University. (in Chinese) |
| 吴琼. 2019. ABA-乙烯互作调控樱桃番茄果实成熟的效应与机理研究[博士论文]. 杭州:浙江大学. | |
| [33] |
Yamaguchi-Shinozaki K, Shinozaki K. 1994. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought,low-temperature,or high-salt stress. Plant Cell, 6 (2):251-264.
pmid: 8148648 |
| [34] |
Yamaguchi-Shinozaki K, Shinozaki K. 2005. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends in Plant Science, 10 (2):88-94.
pmid: 15708346 |
| [35] | Yang W B, Zhang L S, Lv H, Li H, Zhang Y N, Xu Y, Yu J N. 2015. The K-segments of wheat dehydrin WZY 2 are essential for its protective functions under temperature stress. Frontiers Plant Science, 6:406. |
| [36] |
Yang Z, Sheng J Y, Lv K, Ren L, Zhang D. 2019. Y2SK2 and SK3 type dehydrins from Agapanthus praecox can improve plant stress tolerance and act as multifunctional protectants. Plant Science, 284:143-160.
doi: 10.1016/j.plantsci.2019.03.012 URL |
| [37] |
Zhang H F, Liu S Y, Ma J H, Wang X K, Haq S U, Meng Y C, Zhang Y M, Chen R G. 2020. CaDHN4,a salt and cold stress-responsive dehydrin gene from pepper decreases abscisic acid sensitivity in Arabidopsis. International Journal of Molecular Sciences, 21 (1):26.
doi: 10.3390/ijms21010026 URL |
| [38] |
Zhao Y, Wang Y, Liu Q, Zhai Y, Zhao Y, Zhang M J. 2017. Cloning of a new LEA 1 gene promoter from soybean and functional analysis in transgenic tobacco. Plant Cell Tissue and Organ Culture, 130 (4-5):1-13.
doi: 10.1007/s11240-017-1199-2 URL |
| [39] |
Zhu K J, Wu Q J, Huang Y, Ye J L, Xu Q, Deng X X. 2020. Genome-wide characterization of cis-acting elements in the promoters of key carotenoid pathway genes from the main species of genus Citrus. Horticultural Plant Journal, 6 (6):385-395.
doi: 10.1016/j.hpj.2020.10.003 URL |
| [40] |
Zhu W N, Zhang D P, Lu X X, Zhang L S, Yu Z Y, Lv H, Zhang H M. 2014. Characterisation of an SKn-type dehydrin promoter from wheat and its responsiveness to various abiotic and biotic stresses. Plant Molecular Biology Reporter, 32 (3):664-678.
doi: 10.1007/s11105-013-0681-1 URL |
| [1] | YU Tingting, LI Huan, NING Yuansheng, SONG Jianfei, PENG Lulin, JIA Junqi, ZHANG Weiwei, and YANG Hongqiang. Genome-wide Identification of GRAS Gene Family in Apple and Expression Analysis of Its Response to Auxin [J]. Acta Horticulturae Sinica, 2023, 50(2): 397-409. |
| [2] | YUAN Xin, XU Yunhe, ZHANG Yupei, SHAN Nan, CHEN Chuying, WAN Chunpeng, KAI Wenbin, ZHAI Xiawan, CHEN Jinyin, GAN Zengyu. Studies on AcAREB1 Regulating the Expression of AcGH3.1 During Postharvest Ripening of Kiwifruit [J]. Acta Horticulturae Sinica, 2023, 50(1): 53-64. |
| [3] | XU Xiaoping, CAO Qingying, CAI Roudi, GUAN Qingxu, ZHANG Zihao, CHEN Yukun, XU HAN, LIN Yuling, LAI Zhongxiong. Gene Cloning and Expression Analysis of miR408 and Its Target DlLAC12 in Globular Embryo Development and Abiotic Stress in Dimocarpus longan [J]. Acta Horticulturae Sinica, 2022, 49(9): 1866-1882. |
| [4] | JIA Xin, ZENG Zhen, CHEN Yue, FENG Hui, LÜ Yingmin, ZHAO Shiwei. Cloning and Expression Analysis of RcDREB2A Gene in Rosa chinensis‘Old Blush’ [J]. Acta Horticulturae Sinica, 2022, 49(9): 1945-1956. |
| [5] | MA Weifeng, LI Yanmei, MA Zonghuan, CHEN Baihong, MAO Juan. Identification of Apple POD Gene Family and Functional Analysis of MdPOD15 Gene [J]. Acta Horticulturae Sinica, 2022, 49(6): 1181-1199. |
| [6] | WANG Dan, WANG Mi, LIU Jun, ZHOU Xiaohui, LIU Songyu, YANG Yan, ZHUANG Yong. Cloning of U6 Promoters and Establishment of CRISPR/Cas9 Mediated Gene Editing System in Eggplant [J]. Acta Horticulturae Sinica, 2022, 49(4): 791-800. |
| [7] | SONG Fang, LI Zixuan, WANG Ce, WANG Zhijing, HE Ligang, JIANG Yingchun, WU Liming, BAI Fuxi. Cloning and Function Analysis of Mycorrhizal Signaling Receptor Protein Lysin Motif Receptor-like Kinases 2 Gene(LYK2)in Citrus [J]. Acta Horticulturae Sinica, 2022, 49(2): 281-292. |
| [8] | ZHOU Zhiming, YANG Jiabao, ZHANG Cheng, ZENG Linglu, MENG Wanqiu, SUN Li. Genome-wide Identification and Expression Analyses of Long-chain Acyl-CoA Synthetases Under Abiotic Stresses in Helianthus annuus [J]. Acta Horticulturae Sinica, 2022, 49(2): 352-364. |
| [9] | HUANG Renwei, REN Yinghong, QI Weiliang, ZENG Rui, LIU Xinyu, DENG Binyan. Cloning of Mulberry MaERF105-Like Gene and Its Expression Under Drought Stress [J]. Acta Horticulturae Sinica, 2022, 49(11): 2439-2448. |
| [10] | XIE Siyi, ZHOU Chengzhe, ZHU Chen, ZHAN Dongmei, CHEN Lan, WU Zuchun, LAI Zhongxiong, GUO Yuqiong. Genome-wide Identification and Expression Analysis of CsTIFY Transcription Factor Family Under Abiotic Stress and Hormone Treatments in Camellia sinensis [J]. Acta Horticulturae Sinica, 2022, 49(1): 100-116. |
| [11] | LIANG Zhile, WANG Kuanhong, YANG Jing, ZHU Biao, ZHU Zhujun. The Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassicaceae Family [J]. Acta Horticulturae Sinica, 2022, 49(1): 200-220. |
| [12] | MA Junjie, SONG Lina, LI Le, MA Xiaochun, JIN Lei, XU Weirong. VaCBL6 from Vitis amurensis Involved in Abiotic Stress Response and ABA-mediated Pathway [J]. Acta Horticulturae Sinica, 2021, 48(6): 1079-1093. |
| [13] | DENG Zeyi, SONG Xiang, HONG Yan, DAI Silan. Applications of Promoters in the Genetic Engineering of Ornamental Plants:A Review [J]. Acta Horticulturae Sinica, 2021, 48(6): 1250-1264. |
| [14] | CAI Roudi, LI Xue, CHEN Yan, XU Xiaoping, CHEN Xiaohui, LAI Zhongxiong, LIN Yuling. Genome-wide Identification and Expression Analysis of DRB Gene Family in Dimocarpus longan [J]. Acta Horticulturae Sinica, 2021, 48(5): 921-933. |
| [15] | YUE Lingqi, XING Qiaojuan, ZHANG Xiaolan, LIANG Xue, WANG Qian, QI Hongyan. Research Progress on the Effect of Phytochrome-interacting Factors in Plant Resistance to Abiotic Stress [J]. Acta Horticulturae Sinica, 2021, 48(4): 632-646. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2012 Acta Horticulturae Sinica 京ICP备10030308号-2 国际联网备案号 11010802023439
Tel: 010-82109523 E-Mail: yuanyixuebao@126.com
Support by: Beijing Magtech Co.Ltd