
Acta Horticulturae Sinica ›› 2021, Vol. 48 ›› Issue (6): 1053-1066.doi: 10.16420/j.issn.0513-353x.2020-0653
• Research Papers • Next Articles
LIU Jianfeng, SUN Ying, WEI Heng, HE Hongli, ZHANG Xingzheng, CHENG Yunqing( )
)
Received:2021-02-07
Revised:2021-05-11
Online:2021-06-25
Published:2021-07-07
Contact:
CHENG Yunqing
E-mail:Chengyunqing1977@163.com
CLC Number:
LIU Jianfeng, SUN Ying, WEI Heng, HE Hongli, ZHANG Xingzheng, CHENG Yunqing. Analysis and Identification of circRNAs of Hazel Ovule at Different Developmental Stages[J]. Acta Horticulturae Sinica, 2021, 48(6): 1053-1066.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.ahs.ac.cn/EN/10.16420/j.issn.0513-353x.2020-0653
| RNA | 基因名称 Gene ID | 引物序列(5′-3′) Primer sequence | 产物长度/bp Amplicon size |
|---|---|---|---|
| DEcircRNA | 00262:4702|5542 | F:GTTCTGTTCCCTGCCTCCAAT;R:CCATTTTCCCAACATTCCTTAGT | 129 |
| 03593:4109|4340 | F:AAACAAGAGGCCGAAACTAG;R:ATCCATTGTCTGCAAGTGTT | 115 | |
| 05162:7223|7649 | F:CAGTTCAGGGTAGTTGTCG;R:GTTTGCTAGGCTTAGATACTTC | 103 | |
| 05531:13219|13378 | F:TGTGCCCTTCATGTTCTCCTT;R:CACGAGGAGGATTGGAGGAAG | 133 | |
| DEmRNA | g3238 | F:CGGTAATAAGAGCCCAGGTGC;R:CACCTGTGCCGTGAATTTGTC | 142 |
| g18070 | F:CGCTCTTGCTCAGGCTTGTC;R:GAGAAGGCATGGATACCCGTC | 87 | |
| g21729 | F:CTGCAAAGAACTCCCTGTGGTAA;R:TTGGCAGGGCTCAGGCATT | 129 | |
| g22409 | F:GCGGTATCAAGAACCAAAGGG;R:GAGCGTCAAACCAGCAAGTG | 110 | |
| 内参基因 Reference gene | Beta actin | F:CCCTCACAATTTCACGCTCG;R:ATGAGGGTTATGCCCTCCCA | 135 |
Table 1 Primer sequences of DEcircRNAs and their target DEmRNAs in qRT-PCR analysis
| RNA | 基因名称 Gene ID | 引物序列(5′-3′) Primer sequence | 产物长度/bp Amplicon size |
|---|---|---|---|
| DEcircRNA | 00262:4702|5542 | F:GTTCTGTTCCCTGCCTCCAAT;R:CCATTTTCCCAACATTCCTTAGT | 129 |
| 03593:4109|4340 | F:AAACAAGAGGCCGAAACTAG;R:ATCCATTGTCTGCAAGTGTT | 115 | |
| 05162:7223|7649 | F:CAGTTCAGGGTAGTTGTCG;R:GTTTGCTAGGCTTAGATACTTC | 103 | |
| 05531:13219|13378 | F:TGTGCCCTTCATGTTCTCCTT;R:CACGAGGAGGATTGGAGGAAG | 133 | |
| DEmRNA | g3238 | F:CGGTAATAAGAGCCCAGGTGC;R:CACCTGTGCCGTGAATTTGTC | 142 |
| g18070 | F:CGCTCTTGCTCAGGCTTGTC;R:GAGAAGGCATGGATACCCGTC | 87 | |
| g21729 | F:CTGCAAAGAACTCCCTGTGGTAA;R:TTGGCAGGGCTCAGGCATT | 129 | |
| g22409 | F:GCGGTATCAAGAACCAAAGGG;R:GAGCGTCAAACCAGCAAGTG | 110 | |
| 内参基因 Reference gene | Beta actin | F:CCCTCACAATTTCACGCTCG;R:ATGAGGGTTATGCCCTCCCA | 135 |
| 注释数据库Annotation database | 注释数量Annotated number | 基因长度Gene length | |
|---|---|---|---|
| (300 ~ 999 bp) | (> = 1 000 bp) | ||
| COG | 1 440 | 103 | 1 335 |
| GO | 2 398 | 263 | 2 130 |
| KEGG | 1 741 | 188 | 1 548 |
| KOG | 2 377 | 241 | 2 133 |
| Pfam | 2 859 | 243 | 2 616 |
| Swissprot | 2 793 | 280 | 2 509 |
| eggNOG | 3 440 | 367 | 3 068 |
| NR | 3 509 | 391 | 3 113 |
| 总数 All annotated | 3 511 | 391 | 3 115 |
Table 2 Annotation of identified circRNAs
| 注释数据库Annotation database | 注释数量Annotated number | 基因长度Gene length | |
|---|---|---|---|
| (300 ~ 999 bp) | (> = 1 000 bp) | ||
| COG | 1 440 | 103 | 1 335 |
| GO | 2 398 | 263 | 2 130 |
| KEGG | 1 741 | 188 | 1 548 |
| KOG | 2 377 | 241 | 2 133 |
| Pfam | 2 859 | 243 | 2 616 |
| Swissprot | 2 793 | 280 | 2 509 |
| eggNOG | 3 440 | 367 | 3 068 |
| NR | 3 509 | 391 | 3 113 |
| 总数 All annotated | 3 511 | 391 | 3 115 |

Fig. 3 Hierarchical clustering analysis of differentially expressed circRNAs at four developmental stages. A,B and C represent three biological repetitions.
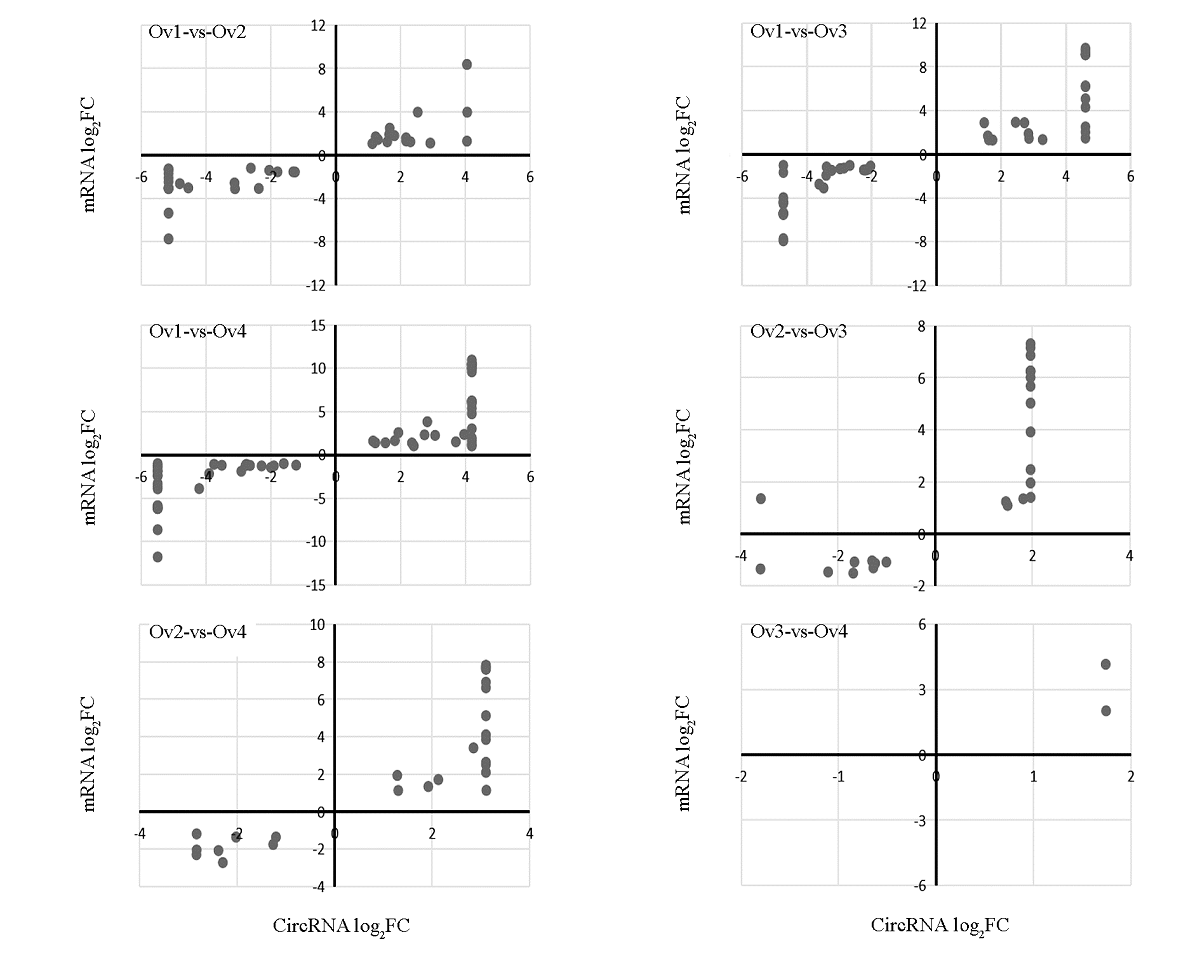
Fig. 4 Correlation analysis of DEcircRNA and their target DEmRNA In paired comparison,FC represents circRNA expression in latter development stage(B)/ circRNA expression in former development stage(A). If B is 0,log2FC = -∞;if A is 0,log2FC = ∞. In order to express each pair of DEcircRNA-DEmRNA in the graph intuitively,when log 2FC = -∞ or ∞,the value of log2FC is recorded as the minimum and maximum value in the paired comparison respectively.

Fig. 6 Analysis of DEcircRNAs and their target DEmRNAs using qRT-PCR Different lowercase letters in the same broken line indicates significant difference at level of P < 0.05.
| 成对比较 Paired comparison | 差异表达circRNA DEcircRNA | log2FC (circRNA) | 差异表达mRNA DEmRNA | log2FC (mRNA) | 注释 Annotation |
|---|---|---|---|---|---|
| Ov1-vs-Ov4 | 19296:1590|1950 | -∞ | Corylus_avellana_newGene_15264 | -11.80 | NPR4 |
| Ov1-vs-Ov2 | 19296:1590|1950 | -∞ | Corylus_avellana_newGene_15264 | -7.74 | NPR4 |
| Ov1-vs-Ov3 | 19296:1590|1950 | -∞ | Corylus_avellana_newGene_15264 | -7.73 | NPR4 |
| Ov1-vs-Ov4 | 00262:4702|5542 | -∞ | g3238 | -6.19 | NPP |
| Ov1-vs-Ov4 | 00262:4702|5735 | -∞ | g3238 | -6.19 | NPP |
| Ov1-vs-Ov4 | 00262:5280|5910 | -∞ | g3238 | -6.19 | NPP |
| Ov1-vs-Ov3 | 01486:5071|5547 | -∞ | g10760 | -4.39 | ABCG9 |
| Ov1-vs-Ov3 | 09164:4340|5637 | -∞ | g27362 | -4.32 | SULTR3;1 |
| Ov1-vs-Ov3 | 05982:1271|3603 | -∞ | Corylus_avellana_newGene_58963 | -3.97 | SCPL18 |
| Ov1-vs-Ov4 | 09164:4340|5637 | -∞ | g27362 | -3.93 | SULTR3;1 |
| Ov1-vs-Ov4 | 05162:7223|7649 | -4.21 | g21729 | -3.90 | SGR |
| Ov1-vs-Ov4 | 05982:1271|3603 | -∞ | Corylus_avellana_newGene_58963 | -3.64 | SCPL18 |
| Ov1-vs-Ov3 | 05162:7223|7649 | -3.48 | g21729 | -3.08 | SGR |
| Ov1-vs-Ov2 | 00262:4702|5542 | -∞ | g3238 | -3.03 | NPP |
| Ov1-vs-Ov2 | 00262:4702|5735 | -4.55 | g3238 | -3.03 | NPP |
| Ov2-vs-Ov4 | 05312:1137|1462 | ∞ | Corylus_avellana_newGene_56641 | 6.62 | NHX4 |
| Ov2-vs-Ov3 | 14244:2582|2731 | ∞ | g31169 | 6.87 | OLE1 |
| Ov2-vs-Ov4 | 01490:2219|2537 | ∞ | g10779 | 6.92 | COR2 |
| Ov2-vs-Ov3 | 03593:4109|4340 | ∞ | g18070 | 7.31 | TIP3-2 |
| Ov2-vs-Ov4 | 03593:4109|4340 | ∞ | g18070 | 7.63 | TIP3-2 |
| 成对比较 Paired comparison | 差异表达circRNA DEcircRNA | log2FC (circRNA) | 差异表达mRNA DEmRNA | log2FC (mRNA) | 注释 Annotation |
| Ov2-vs-Ov4 | 03974:6066|6673 | ∞ | g19072 | 7.80 | SBP |
| Ov2-vs-Ov4 | 14244:2582|2731 | ∞ | g31169 | 7.82 | OLE1 |
| Ov1-vs-Ov2 | 04715:14790|15271 | ∞ | g20792 | 8.37 | WAT1 |
| Ov1-vs-Ov3 | 03593:4109|4340 | ∞ | g18070 | 9.34 | TIP3-2 |
| Ov1-vs-Ov3 | 14244:2582|2731 | ∞ | g31169 | 9.55 | OLE1 |
| Ov1-vs-Ov4 | 03593:4109|4340 | ∞ | g18070 | 9.59 | TIP3-2 |
| Ov1-vs-Ov3 | 05531:13219|13378 | ∞ | g22409 | 9.69 | OLE5 |
| Ov1-vs-Ov4 | 05531:13219|13378 | ∞ | g22409 | 9.99 | OLE5 |
| Ov1-vs-Ov4 | 05531:13219|13433 | ∞ | g22409 | 9.99 | OLE5 |
| Ov1-vs-Ov4 | 14244:2582|2731 | ∞ | g31169 | 10.35 | OLE1 |
Table 3 Thirty pairs of DEcircRNAs with the largest expression fold change and their target DEmRNAs
| 成对比较 Paired comparison | 差异表达circRNA DEcircRNA | log2FC (circRNA) | 差异表达mRNA DEmRNA | log2FC (mRNA) | 注释 Annotation |
|---|---|---|---|---|---|
| Ov1-vs-Ov4 | 19296:1590|1950 | -∞ | Corylus_avellana_newGene_15264 | -11.80 | NPR4 |
| Ov1-vs-Ov2 | 19296:1590|1950 | -∞ | Corylus_avellana_newGene_15264 | -7.74 | NPR4 |
| Ov1-vs-Ov3 | 19296:1590|1950 | -∞ | Corylus_avellana_newGene_15264 | -7.73 | NPR4 |
| Ov1-vs-Ov4 | 00262:4702|5542 | -∞ | g3238 | -6.19 | NPP |
| Ov1-vs-Ov4 | 00262:4702|5735 | -∞ | g3238 | -6.19 | NPP |
| Ov1-vs-Ov4 | 00262:5280|5910 | -∞ | g3238 | -6.19 | NPP |
| Ov1-vs-Ov3 | 01486:5071|5547 | -∞ | g10760 | -4.39 | ABCG9 |
| Ov1-vs-Ov3 | 09164:4340|5637 | -∞ | g27362 | -4.32 | SULTR3;1 |
| Ov1-vs-Ov3 | 05982:1271|3603 | -∞ | Corylus_avellana_newGene_58963 | -3.97 | SCPL18 |
| Ov1-vs-Ov4 | 09164:4340|5637 | -∞ | g27362 | -3.93 | SULTR3;1 |
| Ov1-vs-Ov4 | 05162:7223|7649 | -4.21 | g21729 | -3.90 | SGR |
| Ov1-vs-Ov4 | 05982:1271|3603 | -∞ | Corylus_avellana_newGene_58963 | -3.64 | SCPL18 |
| Ov1-vs-Ov3 | 05162:7223|7649 | -3.48 | g21729 | -3.08 | SGR |
| Ov1-vs-Ov2 | 00262:4702|5542 | -∞ | g3238 | -3.03 | NPP |
| Ov1-vs-Ov2 | 00262:4702|5735 | -4.55 | g3238 | -3.03 | NPP |
| Ov2-vs-Ov4 | 05312:1137|1462 | ∞ | Corylus_avellana_newGene_56641 | 6.62 | NHX4 |
| Ov2-vs-Ov3 | 14244:2582|2731 | ∞ | g31169 | 6.87 | OLE1 |
| Ov2-vs-Ov4 | 01490:2219|2537 | ∞ | g10779 | 6.92 | COR2 |
| Ov2-vs-Ov3 | 03593:4109|4340 | ∞ | g18070 | 7.31 | TIP3-2 |
| Ov2-vs-Ov4 | 03593:4109|4340 | ∞ | g18070 | 7.63 | TIP3-2 |
| 成对比较 Paired comparison | 差异表达circRNA DEcircRNA | log2FC (circRNA) | 差异表达mRNA DEmRNA | log2FC (mRNA) | 注释 Annotation |
| Ov2-vs-Ov4 | 03974:6066|6673 | ∞ | g19072 | 7.80 | SBP |
| Ov2-vs-Ov4 | 14244:2582|2731 | ∞ | g31169 | 7.82 | OLE1 |
| Ov1-vs-Ov2 | 04715:14790|15271 | ∞ | g20792 | 8.37 | WAT1 |
| Ov1-vs-Ov3 | 03593:4109|4340 | ∞ | g18070 | 9.34 | TIP3-2 |
| Ov1-vs-Ov3 | 14244:2582|2731 | ∞ | g31169 | 9.55 | OLE1 |
| Ov1-vs-Ov4 | 03593:4109|4340 | ∞ | g18070 | 9.59 | TIP3-2 |
| Ov1-vs-Ov3 | 05531:13219|13378 | ∞ | g22409 | 9.69 | OLE5 |
| Ov1-vs-Ov4 | 05531:13219|13378 | ∞ | g22409 | 9.99 | OLE5 |
| Ov1-vs-Ov4 | 05531:13219|13433 | ∞ | g22409 | 9.99 | OLE5 |
| Ov1-vs-Ov4 | 14244:2582|2731 | ∞ | g31169 | 10.35 | OLE1 |
| [1] |
Ambros V R. 2004. The functions of animal microRNAs. Nature, 431:350-355.
doi: 10.1038/nature02871 URL |
| [2] | An Lin-jun, Luan Jia-yu, Ren Li, Li Hui-yu, Xia De-an. 2019. Bioinformatics and expression characteristics analysis of BpTCP8 in Betula platyphylla Suk. Journal of Nanjing Forestry Univeristy(Natural Sciences Edition), 43:67-73. (in Chinese) |
| 安琳君, 栾嘉豫, 任丽, 李慧玉, 夏德安. 2019. 白桦 BpTCP8基因生物信息学及表达特性分析. 南京林业大学学报(自然科学版), 43:67-73. | |
| [3] |
Apweiler R, Bairoch A M, Wu C H, Barker W C, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M. 2004. UniProt:the universal protein knowledgebase. Nucleic Acids Research, 32:115-119.
doi: 10.1093/nar/gkh151 URL |
| [4] |
Ashburner M, Ball C A, Blake J A, Botstein D, Butler H, Cherry J M, Davis A P, Dolinski K, Dwight S S, Eppig J T, Harris M A, Hill D P, Issel-Tarver L, Kasarskis A, Lewis S, Matese J C, Richardson J E, Ringwald M, Rubin G M, Sherlock G. 2000. Gene ontology:tool for the unification of biology. Nature Genetics, 25:25-29.
doi: 10.1038/75556 URL |
| [5] |
Ashwalfluss R, Meyer M, Pamudurti N R, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. 2014. circRNA biogenesis competes with pre-mRNA splicing. Molecular Cell, 56:55-66.
doi: 10.1016/j.molcel.2014.08.019 URL |
| [6] |
Bartel D P. 2004. microRNAs:genomics,biogenesis,mechanism,and function. Cell, 116:281-297.
pmid: 14744438 |
| [7] |
Bartel D P. 2009. microRNAs:target recognition and regulatory functions. Cell, 136:215-233.
doi: 10.1016/j.cell.2009.01.002 pmid: 19167326 |
| [8] |
Bartel D P. 2018. Metazoan microRNAs. Cell, 173:20-51.
doi: S0092-8674(18)30286-1 pmid: 29570994 |
| [9] |
Cao H P, Zhang L, Tan X F, Long H X, Shockey J M. 2014. Identification,classification and differential expression of oleosin genes in tung tree (Vernicia fordii). PLoS ONE, 9:e88409.
doi: 10.1371/journal.pone.0088409 URL |
| [10] |
Cao M J, Wang Z, Wirtz M, Hell R, Oliver D J, Xiang C B. 2013. SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant Journal, 73:607-616.
doi: 10.1111/tpj.2013.73.issue-4 URL |
| [11] | Cheng Yun-qing, Zhang Li-na, Zhao Yong-bin, Liu Jian-feng. 2018. Analysis of SSR markers information and primer selection from transcriptome sequence of hybrid hazelnut Corylus heterophylla × C. avellana. Acta Horticulturae Sinica, 45 (1):139-148. (in Chinese) |
| 程云清, 张丽娜, 赵永斌, 刘剑锋. 2018. 平欧杂交榛转录组中SSR信息分析和引物筛选. 园艺学报, 45 (1):139-148. | |
| [12] | Deng Yang-yang, Li Jian-qi, Wu Song-feng, Zhu Yun-ping, Chen Yao-wen, He Fu-chu. 2006. Integrated nr database in protein annotation system and its localization. Computer Engineering, 32:71-74. (in Chinese) |
| 邓泱泱, 荔建琦, 吴松锋, 朱云平, 陈耀文, 贺福初. 2006. nr数据库分析及其本地化. 计算机工程, 32:71-73. | |
| [13] |
Eddy S R. 1998. Profile hidden Markov models. Bioinformatics, 14:755-763.
pmid: 9918945 |
| [14] | Gao Zhen, Luo Meng, Wang Lei, Song Shiren, Zhao Liping, Xu Wenping, Zhang Caixi, Wang Shiping, Ma Chao. 2019. The research advance of plant circular RNA. Acta Horticulturae Sinica, 46 (1):171-181. |
| 高振, 骆萌, 王磊, 宋士任, 赵丽萍, 许文平, 张才喜, 王世平, 马超. 2019. 植物环状RNA研究进展. 园艺学报, 46 (1):171-181. | |
| [15] |
Hansen T B, Jensen T I, Clausen B H, Bramsen J B, Finsen B, Damgaard C K, Kjems J. 2013. Natural RNA circles function as efficient microRNA sponges. Nature, 495:384-388.
doi: 10.1038/nature11993 URL |
| [16] | Hu Xiao-yi, Tan Xiao-feng, Tian Xiao-ming, Liu Qiao, Luo Qian, Chen Hong-peng, Hu Fang-min. 2008. Identification and analysis of an aquaporin (CoPIP1-1)in the seeds of Camellia oleifer. Scientia Silvae Sinicae, 44:48-56. (in Chinese) |
| 胡孝义, 谭晓风, 田晓明, 刘巧, 罗茜, 陈鸿鹏, 胡芳名. 2008. 油茶种子水通道蛋白CoPIP1-1的鉴定与分析. 林业科学, 44:51-59. | |
| [17] |
Jeck W R, Sharpless N E. 2014. Detecting and characterizing circular RNAs. Nature Biotechnology, 32:453-461.
doi: 10.1038/nbt.2890 URL |
| [18] | Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. 2004. The KEGG resource for deciphering the genome. Nucleic Acids Research, 32:277-280. |
| [19] | Koonin E V, Fedorova N D, Jackson J D, Jacobs A R, Krylov D M, Makarova K S, Mazumder R, Mekhedov S L, Nikolskaya A N, Rao B S. 2004. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biology, 5:1-28. |
| [20] |
Kristensen L S, Andersen M S, Stagsted L V W, Ebbesen K K, Hansen T B, Kjems J. 2019. The biogenesis,biology and characterization of circular RNAs. Nature Reviews Genetics, 20:675-691.
doi: 10.1038/s41576-019-0158-7 |
| [21] |
Le Hir R, Sorin C, Chakraborti D, Moritz T, Schaller H, Tellier F, Robert S, Morin H, Bako L, Bellini C. 2013. ABCG9,ABCG11 and ABCG14 ABC transporters are required for vascular development in Arabidopsis. Plant Journal, 76:811-824.
doi: 10.1111/tpj.12334 URL |
| [22] |
Li B, Ruotti V, Stewart R M, Thomson J A, Dewey C N. 2010. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics, 26:493-500.
doi: 10.1093/bioinformatics/btp692 URL |
| [23] | Li J Q, Yang J, Zhou P, Le Y P, Zhou C W, Wang S M, Xu D Z, Lin H K, Gong Z H. 2015a. Circular RNAs in cancer:novel insights into origins,properties,functions and implications. American Journal of Cancer Research, 5:472-480. |
| [24] |
Li Y B, Fan C C, Xing Y Z, Jiang Y H, Luo L J, Sun L, Shao D, Xu C J, Li X H, Xiao J H. 2011. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nature Genetics, 43:1266-1269.
doi: 10.1038/ng.977 URL |
| [25] |
Li Z Y, Huang C, Bao C, Chen L, Lin M, Wang X L, Zhong G L, Yu B, Hu W C, Dai L M. 2015b. Exon-intron circular RNAs regulate transcription in the nucleus. Nature Structural & Molecular Biology, 22:256-264.
doi: 10.1038/nsmb.2959 URL |
| [26] |
Liu J F, Cheng Y Q, Yan K, Liu Q, Wang Z W. 2012. The relationship between reproductive growth and blank fruit formation in Corylus heterophylla Fisch. Scientia Horticulturae, 136:128-134.
doi: 10.1016/j.scienta.2012.01.008 URL |
| [27] |
Liu J F, Luo Q Z, Zhang X Z, Zhang Q, Cheng Y Q. 2020. Identification of vital candidate microRNA/mRNA pairs regulating ovule development using high-throughput sequencing in hazel. BMC Developmental Biology, 20:13.
doi: 10.1186/s12861-020-00219-z URL |
| [28] |
Liu J F, Zhang H D, Cheng YQ, Kafkas S, Guney M. 2014. Pistillate flower development and pollen tube growth mode during the delayed fertilization stage in Corylus heterophylla Fisch. Plant Reproduction, 27:145-152.
doi: 10.1007/s00497-014-0248-9 URL |
| [29] |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25:402-408.
pmid: 11846609 |
| [30] | Lü Dong-lin, Guo Yi-wen, Han Rui, Jiang Jing. 2018. Characterization of gene expression in anthocyanin synthesisand salt tolerance of Betula pendula 'Purple Rain'. Journal of Nanjing Forestry Univeristy(Natural Sciences Edition), 42:25-32. (in Chinese) |
| 吕东林, 林琳, 郭译文, 韩锐, 姜静. 2018. 紫雨桦耐盐性及花色苷合成相关基因的表达特性. 南京林业大学学报(自然科学版), 42:28-35. | |
| [31] |
Ma L, Li T, Hao C Y, Wang Y Q, Chen X H, Zhang X Y. 2016. TaGS5-3A,a grain size gene selected during wheat improvement for larger kernel and yield. Plant Biotechnology Journal, 14:1269-1280.
doi: 10.1111/pbi.2016.14.issue-5 URL |
| [32] |
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak S D, Gregersen L H, Munschauer M. 2013. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature, 495:333-338.
doi: 10.1038/nature11928 URL |
| [33] |
Nanjo Y, Oka H, Ikarashi N, Kaneko K, Kitajima A, Mitsui T, Munoz F, Rodriguezlopez M, Barojafernandez E, Pozuetaromero J. 2006. Rice plastidial N-glycosylated nucleotide pyrophosphatase/phosphodiesterase is transported from the ER-golgi to the chloroplast through the secretory pathway. Plant Cell, 18:2582-2592.
doi: 10.1105/tpc.105.039891 URL |
| [34] |
Philips A, Nowis K, Stelmaszczuk M, Podkowiński J, Handschuh L, Jackowiak P, Figlerowicz M. 2020. Arabidopsis thaliana cbp80, c2h2,and flk knockout mutants accumulate increased amounts of circular RNAs. Cells, 9:1937.
doi: 10.3390/cells9091937 URL |
| [35] |
Ranocha P, Dima O, Nagy R, Felten J, Corratgé-Faillie C, Novák O, Morreel K, Lacombe B, Martinez Y, Pfrunder S, Jin X, Renou J P, Thibaud J B, Ljung K, Fischer U, Martinoia E, Boerjan W, Goffner D. 2013. Arabidopsis WAT 1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nature Communications, 4:2625.
doi: 10.1038/ncomms3625 URL |
| [36] | Shi Tian-pei, Wang Xin-yue, Hou Hao-bin, Zhao Zhi-da, Shang Ming-yu, Zhang Li. 2020. Analysis and identification of circRNAs of skeletal muscle at different stages of sheep embryos based on whole transcriptome sequencing. Scientia Agricultura Sinica, 53:642-657. (in Chinese) |
| 石田培, 王欣悦, 侯浩宾, 赵志达, 尚明玉, 张莉. 2020. 基于全转录组测序的绵羊胚胎不同发育阶段骨骼肌circRNA的分析与鉴定. 中国农业科学, 53:642-657. | |
| [37] |
Tan J J, Zhou Z J, Niu Y J, Sun X Y, Deng Z P. 2017. Identification and functional characterization of tomato circRNAs derived from genes involved in fruit pigment accumulation. Scientific Reports, 7:8594.
doi: 10.1038/s41598-017-08806-0 URL |
| [38] | Tatusov R L, Galperin M Y, Natale D A, Koonin E V. 2000. The COG database:a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Research, 28:33-36. |
| [39] |
Tay Y, Rinn J L, Pandolfi P P. 2014. The multilayered complexity of ceRNA crosstalk and competition. Nature, 505:344-352.
doi: 10.1038/nature12986 URL |
| [40] | Tian Xue-yao, Zhou Jie, Wang Bao-song, He Kai-yue, He Xu-dong. 2020. Cloning and expression pattern analysis of NAC genes in Salix. Journal of Nanjing Forestry Univeristy(Natural Sciences Edition), 44:123-128. (in Chinese) |
| 田雪瑶, 周洁, 王保松, 何开跃, 何旭东. 2020. 柳树 NAC基因的克隆与表达模式分析. 南京林业大学学报(自然科学版), 44:123-128. | |
| [41] | Wang H L, Xiao Y, Wu L, Ma D C. 2018. Comprehensive circular RNA profiling reveals the regulatory role of the circRNA-000911/miR-449a pathway in breast carcinogenesis. International Journal of Oncology, 52:743-754. |
| [42] |
Wang Y, Xiong Z Y, Li Q, Sun Y Y, Jin J, Chen H, Zou Y, Huang X G, Ding Y. 2019. Circular RNA profiling of the rice photo-thermosensitive genic male sterile line Wuxiang S reveals circRNA involved in the fertility transition. BMC Plant Biology, 19:340.
doi: 10.1186/s12870-019-1944-2 URL |
| [43] |
Wang Y X, Wang Q, Gao L P, Zhu B Z, Luo Y B, Deng Z P, Zuo J H. 2017a. Integrative analysis of circRNAs acting as ceRNAs involved in ethylene pathway in tomato. Physiologia Plantarum, 161:311-321.
doi: 10.1111/ppl.2017.161.issue-3 URL |
| [44] | Wang Z P, Liu Y F, Li D W, Li L, Zhang Q, Wang S B, Huang H W. 2017b. Identification of circular RNAs in kiwifruit and their species-specific response to bacterial canker pathogen invasion. Frontiers in Plant Science, 8:413. |
| [45] | Xiong H, Shekhar S, Tan P N, Kumar V. 2004. Exploiting a support-based upper bound of Pearson's correlation coefficient for efficiently identifying strongly correlated pairs//Proceedings of the tenth ACM SIGKDD international conference on knowledge discovery and data mining. Seattle:Association for Computing Machinery:334-343. |
| [46] |
Xu C J, Liu Y, Li Y B, Xu X D, Xu C G, Li X H, Xiao J H, Zhang Q F. 2015. Differential expression of GS5 regulates grain size in rice. Journal of Experimental Botany, 66:2611-2623.
doi: 10.1093/jxb/erv058 URL |
| [47] |
Yao J T, Zhao S H, Liu Q P, Lü M Q, Zhou D X, Liao Z J, Nan K J. 2017. Over-expression of CircRNA_100876 in non-small cell lung cancer and its prognostic value. Pathology-Research and Practice, 213:453-456.
doi: 10.1016/j.prp.2017.02.011 URL |
| [48] |
Zhang Y, Zhang X O, Chen T, Xiang J F, Yin Q F, Xing Y H, Zhu S S, Yang L, Chen L L. 2013. Circular intronic long noncoding RNAs. Molecular Cell, 51:792-806.
doi: 10.1016/j.molcel.2013.08.017 URL |
| [49] |
Zhao W, Cheng Y H, Zhang C, You Q B, Shen X J, Guo W, Jiao Y Q. 2017. Genome-wide identification and characterization of circular RNAs by high throughput sequencing in soybean. Scientific Reports, 7:5636.
doi: 10.1038/s41598-017-05922-9 URL |
| [50] |
Zhou R, Yu X Q, Ottosen C O, Zhao T M. 2020. High throughput sequencing of CircRNAs in tomato leaves responding to multiple stresses of drought and heat. Horticultural Plant Journal, 6 (1):34-38.
doi: 10.1016/j.hpj.2019.12.004 URL |
| [1] | JIANG Yu, TU Xunliang, and HE Junrong. Analysis of Differential Expression Genes in Leaves of Leaf Color Mutant of Chinese Orchid [J]. Acta Horticulturae Sinica, 2023, 50(2): 371-381. |
| [2] | ZHAO Xueyan, WANG Qi, WANG Li, WANG Fangyuan, WANG Qing, LI Yan. Comparative Transcriptome Analysis of Differential Expression in Different Tissues of Corydalis yanhusuo [J]. Acta Horticulturae Sinica, 2023, 50(1): 177-187. |
| [3] | ZHOU Xuzixin, YANG Wei, MAO Meiqin, XUE Yanbin, MA Jun. Identification of Pigment Components and Key Genes in Carotenoid Pathway in Mutants of Chimeric Ananas comosus var. bracteatus [J]. Acta Horticulturae Sinica, 2022, 49(5): 1081-1091. |
| [4] | SHEN Nan, ZHANG Jingcheng, WANG Chengchen, BIAN Yinbing, XIAO Yang. Studies on Transcriptome During Fruiting Body Development of Lentinula edodes [J]. Acta Horticulturae Sinica, 2022, 49(4): 801-815. |
| [5] | XIA Ming, LI Jingwei, LUO Zhangrui, ZU Guidong, WANG Ya, ZHANG Wanping. Research on Mechanism of Exogenous Melatonin Effects on Radish Growth and Resistence to Alternaria brassicae [J]. Acta Horticulturae Sinica, 2022, 49(3): 548-560. |
| [6] | DENG Jiao, SU Mengyue, LIU Xuelian, OU Kefang, HU Zhengrong, YANG Pingfang. Transcriptome Analysis Revealed the Formation Mechanism of Floral Color of Lotus‘Dasajin’with Bicolor Petal [J]. Acta Horticulturae Sinica, 2022, 49(2): 365-377. |
| [7] | QIAO Jun, WANG Liying, LIU Jing, LI Suweng. Expression Analysis of Genes Related to Photosensitive Color Under the Caylx in Eggplant Based on Transcriptome Sequencing [J]. Acta Horticulturae Sinica, 2022, 49(11): 2347-2356. |
| [8] | WANG Ronghua, WANG Shubin, LIU Shuantao, LI Qiaoyun, ZHANG Zhigang, WANG Lihua, ZHAO Zhizhong. Transcriptome Analysis of Waxy Near-isogenic Lines in Chinese Cabbage Floral Axis [J]. Acta Horticulturae Sinica, 2022, 49(1): 62-72. |
| [9] | XU Hongxia, ZHOU Huifen, LI Xiaoying, JIANG Luhua, CHEN Junwei. Comparative Transcriptome Analysis of Different Developmental Stages of Flowers and Fruits in Loquat Under Low Temperature Stress [J]. Acta Horticulturae Sinica, 2021, 48(9): 1680-1694. |
| [10] | LAN Liming, LUO Changguo, WANG Sanhong. Analysis of Resistance Mechanism to Powdery Mildew Based on Transcriptome Sequencing in Malus hupehensis [J]. Acta Horticulturae Sinica, 2021, 48(5): 860-872. |
| [11] | YU Shangqi, ZHANG Rui, GUO Zhongzhong, SONG Yan, FU Jiazhi, WU Pengyu, MA Zhihao. Dynamic Changes of Auxin and Analysis of Differentially Expressed Genes in Walnut Endocarp During Hardening [J]. Acta Horticulturae Sinica, 2021, 48(3): 487-504. |
| [12] | GUO Jun1,2,ZHU Jie1,2,XIE Shangqian1,3,ZHANG Ye1,2,YE Beilei1,2,ZHENG Liyan2,and LING Peng1,3,* . Development of SSR Molecular Markers Based on Transcriptome and Analysis of Genetic Relationship of Germplasm Resources in Avocado [J]. ACTA HORTICULTURAE SINICA, 2020, 47(8): 1552-1564. |
| [13] | XU Junxu1,LI Qingzhu1,LI Ye2,YANG Liuyan1,LI Xin1,WANG Zhen1,and ZHANG Yongchun1,*. Differential Expression of Genes Related to Endogenous Hormone During Bulb Development in Lycoris radiata [J]. ACTA HORTICULTURAE SINICA, 2020, 47(6): 1126-1140. |
| [14] | JU Lixiang,LEI Xin,ZHAO Chengzhi,SHU Huangying,WANG Zhiwei,and CHENG Shanhan*. Identification of MYB Family Genes and Its Relationship with Pungency of Pepper [J]. ACTA HORTICULTURAE SINICA, 2020, 47(5): 875-892. |
| [15] | LI Yanlong,ZHANG Huamin*,CUI Yungang,CHEN Jianhua,Lü Aiqin,LI Jijun,and LI Yixiao. Analysis on SSR Information in Full-length Transcriptome and Development of Molecular Markers in Allium tuberosum [J]. ACTA HORTICULTURAE SINICA, 2020, 47(4): 759-768. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2012 Acta Horticulturae Sinica 京ICP备10030308号-2 国际联网备案号 11010802023439
Tel: 010-82109523 E-Mail: yuanyixuebao@126.com
Support by: Beijing Magtech Co.Ltd