
园艺学报 ›› 2022, Vol. 49 ›› Issue (6): 1275-1289.doi: 10.16420/j.issn.0513-353x.2021-0306
孟宪敏1,2, 崔青青1, 段韫丹1, 庄团结1, 濮丹1, 董春娟1, 杨文才2, 尚庆茂1,*( )
)
收稿日期:2021-11-30
修回日期:2022-02-18
出版日期:2022-06-25
发布日期:2022-07-04
通讯作者:
尚庆茂
E-mail:shangqingmao@caas.cn
基金资助:
MENG Xianmin1,2, CUI Qingqing1, DUAN Yundan1, ZHUANG Tuanjie1, PU Dan1, DONG Chunjuan1, YANG Wencai2, SHANG Qingmao1,*( )
)
Received:2021-11-30
Revised:2022-02-18
Online:2022-06-25
Published:2022-07-04
Contact:
SHANG Qingmao
E-mail:shangqingmao@caas.cn
摘要:
烯效唑(Uniconazole,S3307)作为赤霉素抑制剂,常用于调控植物生长发育,但是否影响嫁接愈合,目前尚不清楚。以番茄‘硬粉8号’为接穗材料,以‘砧爱1号’为砧木材料,于播种后15和21 d分两次叶面喷施0.25 mg · L-1 烯效唑,结果发现烯效唑处理抑制了接穗和砧木幼苗生长,至播种后27 d嫁接时,株高分别较去离子水处理的对照降低了20.58%和12.75%;嫁接后12 ~ 72 h,烯效唑处理降低了嫁接接合部GA15和GA8含量,促进了GA3、IAA、MEIAA、ICAld、TRP和iP7G积累;增加了接合部上方与下方IAA1相对表达量的差异,上调了接合部上方GA20ox1、IAA1和ARR17的相对表达量,下调了接合部下方GA20ox1和ARR17相对表达量。嫁接后6 ~ 168 h,烯效唑处理提高了嫁接接合部TMO6和CyclinB1;2的相对表达量,以及接合部上方WIND1、WOX4和VND7的表达量;使嫁接接合部上方与下方IAA1、ARR17、WIND1、TMO6、WOX4和VND7的表达更早趋于一致,木质部和韧皮部连接提前。另外,烯效唑处理的幼苗嫁接接合部于嫁接后72 h开始连接,嫁接后96 ~ 120 h木质部和韧皮部连接基本完成,早于对照24 h。表明番茄幼苗嫁接前叶面喷施烯效唑可有效调节嫁接接合部内源激素水平,促进嫁接愈合。
中图分类号:
孟宪敏, 崔青青, 段韫丹, 庄团结, 濮丹, 董春娟, 杨文才, 尚庆茂. 烯效唑对番茄幼苗嫁接愈合的促进作用及其机理研究[J]. 园艺学报, 2022, 49(6): 1275-1289.
MENG Xianmin, CUI Qingqing, DUAN Yundan, ZHUANG Tuanjie, PU Dan, DONG Chunjuan, YANG Wencai, SHANG Qingmao. Promoting Effects of Uniconazole on Grafting Formation of Tomato Seedlings and Underlying Mechanisms[J]. Acta Horticulturae Sinica, 2022, 49(6): 1275-1289.
| 基因名称 Gene | 引物序列(5′-3′) Primer sequence |
|---|---|
| GA20ox1 | F:TGGACGATGAATGGCGTTCC;R:TACCGCTCTGTGTAGGCAAC |
| IAA1 | F:TGGATGGTGCCCCTTATCTA;R:ACAAGAAGACATAAACATTTCCCAA |
| ARABIDOPSIS RESPONSE REGULATOR17,ARR17 | F:TGGACGATGAATGGCGTTCC;R:TACCGCTCTGTGTAGGCAAC |
| WOUND INDUCED DEDIFFERENTIATION1,WIND1 | F:AACTGGTACTGCCAACTCCG;R:AGTCCAAGCGTGAACCCAAG |
| TARGET of MONOPTEROS6,TMO6 | F:AAGACGTGTAGGAGGTACTGGA;R:GATAAATTCGGTATCTGCGGCG |
| WUSCHEL-RELATED HOMEOBOX4,WOX4 | F:GTGGAATCACACCCAGGAGG;R:TATTTGTTGCGCGTTGGGTG |
| Cyclin B1;2 | F:GGAAAGCCGCTTCCTCAAGT;R:GCTCCGTTAGCAACAATCGG |
| VASCULAR-RELATED NAC-DOMAIN7,VND7 | F:TAGGACCAACCGAGCCACT;R:TCCGCATTCCGATGACACT |
| NAC DOMAIN-CONTAINING PROTEIN20,NAC020 | F:TGGACGATGAATGGCGTTCC;R:TACCGCTCTGTGTAGGCAAC |
| Actin | F:AACAACGCCTCTTTCTTCTCTCT;R:AAAGAGATCCACAACCACTGTCT |
表1 引物设计
Table 1 Primer sequences
| 基因名称 Gene | 引物序列(5′-3′) Primer sequence |
|---|---|
| GA20ox1 | F:TGGACGATGAATGGCGTTCC;R:TACCGCTCTGTGTAGGCAAC |
| IAA1 | F:TGGATGGTGCCCCTTATCTA;R:ACAAGAAGACATAAACATTTCCCAA |
| ARABIDOPSIS RESPONSE REGULATOR17,ARR17 | F:TGGACGATGAATGGCGTTCC;R:TACCGCTCTGTGTAGGCAAC |
| WOUND INDUCED DEDIFFERENTIATION1,WIND1 | F:AACTGGTACTGCCAACTCCG;R:AGTCCAAGCGTGAACCCAAG |
| TARGET of MONOPTEROS6,TMO6 | F:AAGACGTGTAGGAGGTACTGGA;R:GATAAATTCGGTATCTGCGGCG |
| WUSCHEL-RELATED HOMEOBOX4,WOX4 | F:GTGGAATCACACCCAGGAGG;R:TATTTGTTGCGCGTTGGGTG |
| Cyclin B1;2 | F:GGAAAGCCGCTTCCTCAAGT;R:GCTCCGTTAGCAACAATCGG |
| VASCULAR-RELATED NAC-DOMAIN7,VND7 | F:TAGGACCAACCGAGCCACT;R:TCCGCATTCCGATGACACT |
| NAC DOMAIN-CONTAINING PROTEIN20,NAC020 | F:TGGACGATGAATGGCGTTCC;R:TACCGCTCTGTGTAGGCAAC |
| Actin | F:AACAACGCCTCTTTCTTCTCTCT;R:AAAGAGATCCACAACCACTGTCT |
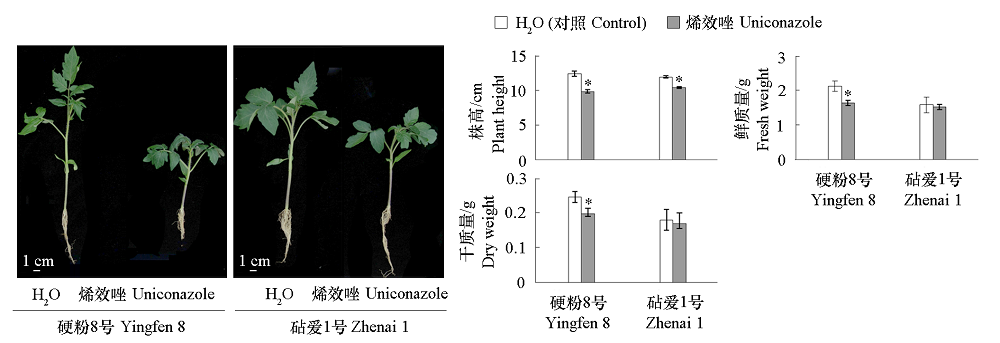
图1 烯效唑处理的番茄接穗品种‘硬粉8号’和砧木品种‘砧爱1号’幼苗生长状况 *表示0.05水平差异显著,数据采用IBM SPSS Statistics 20.0软件进行独立样本T检验。下同。
Fig. 1 Exogenous uniconazole inhibited the growth of tomato scion and stock seedlings * indicates the significance of 0.05 level difference,IBM SPSS Statistics 20.0 software was used for independent-sample T-test. The same below.

图2 番茄接穗(Sc)和砧木(St)幼苗嫁接后48 ~ 168 h接合部纵切面显微结构 pc:薄壁细胞;co:皮层;vb:维管束;ph:韧皮部;x:木质部;vc:维管束连接;两个红色短划线中间区域为嫁接接合部。
Fig. 2 Exogenous uniconazole promoted graft formation process of tomato graft union Sc:Scion;St:Stock;pc:Parenchyma cell;co:Cortex;vb:Vascular bundle;ph:Phloem;x:Xylem;vc:Vascular bundle connection;The middle area of two red dashes is graft union.

图3 番茄嫁接接合部木质部、韧皮部连接显微观察 A. 木质部重连,徒手横切接合部上方(接穗)1 cm处,于体视显微镜观测酸性品红吸收含量。B. 韧皮部重连,徒手横切接合部下方(砧木)1 cm处,于荧光显微镜下观测荧光强度。
Fig. 3 Exogenous uniconazole promoted xylem and phloem connection of tomato graft union A indicates xylem reconnection,which shows the hand-cut transverse section of 1 cm above the graft union(scion),and observed the absorption content of acid fuchsin under stereomicroscope. B indicates phloem reconnection,the hand-cut transverse section of 1 cm below the graft union(stock),and observed the fluorescence intensity under the fluorescence imaging microscope.
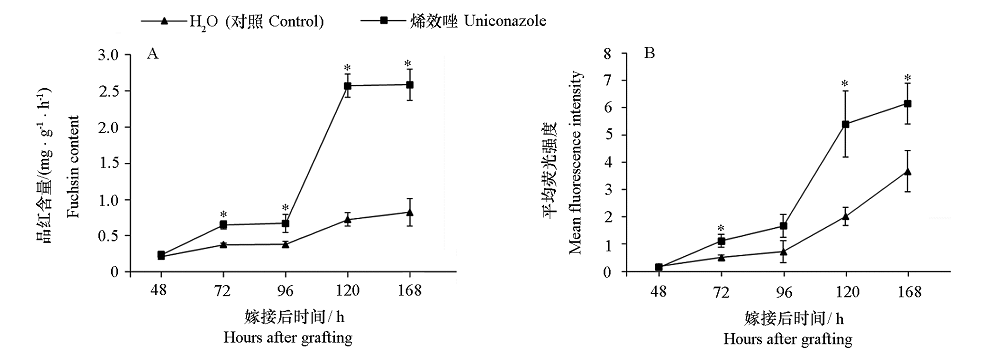
图4 番茄嫁接接合部木质部、韧皮部连接品红含量和荧光强度 A. 表示木质部重连,嫁接接合部上方全部真叶的酸性品红吸收含量;B. 韧皮部重连,徒手横切接合部下方(砧木)1 cm处,于荧光显微镜下观测荧光强度。* P < 0.05。
Fig. 4 Exogenous uniconazole promoted xylem and phloem connection of tomato graft union A indicates xylem reconnection,and observed the absorption content of acid fuchsin under stereomicroscope. B indicates phloem reconnection,the hand-cut transverse section of 1 cm below the graft union(stock),and observed the fluorescence intensity under the fluorescence imaging microscope. * P < 0.05.
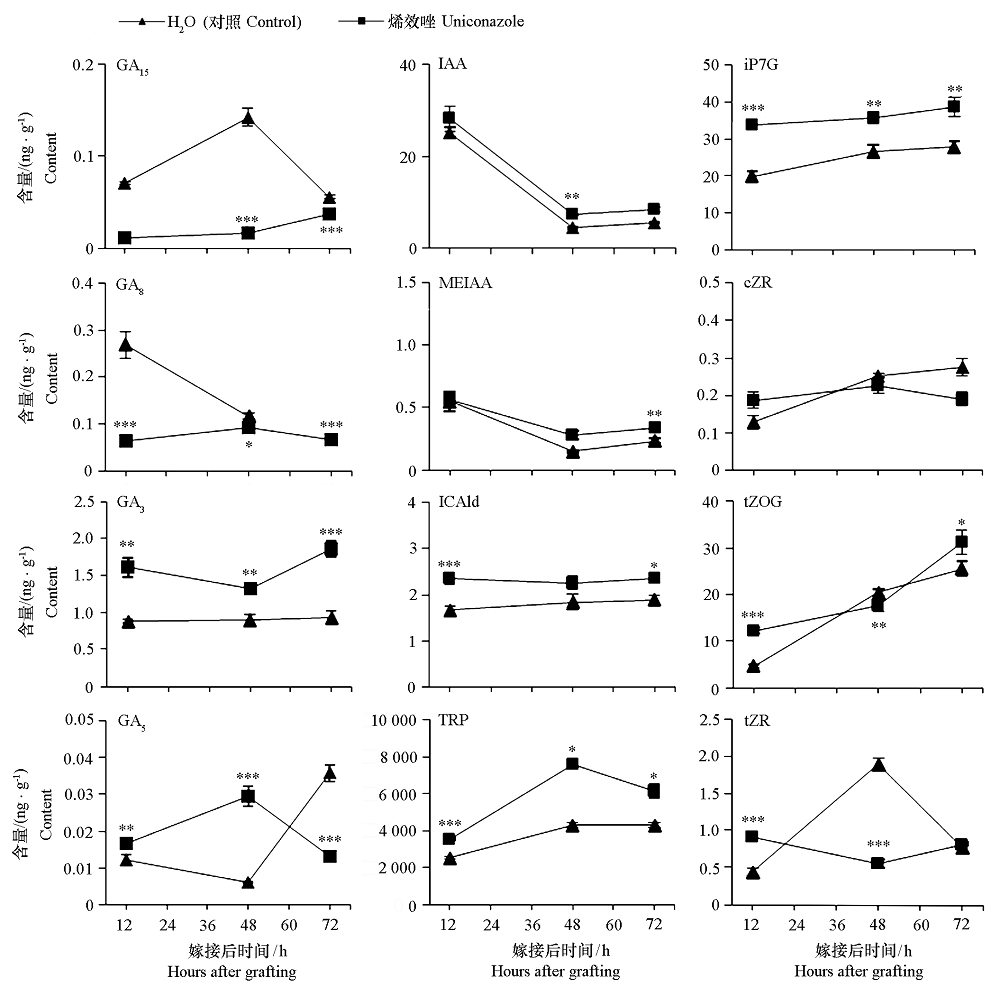
图5 番茄嫁接接合部植物激素含量变化 GA15、GA8、GA3和GA5分别为赤霉素15、赤霉素8、赤霉素3和赤霉素5,GA8部分数据由于样品中激素含量低于仪器的检出限,未检测出该物质;IAA、MEIAA、ICAld和TRP分别为吲哚-3-乙酸、吲哚-3-乙酸甲酯、吲哚-3-甲醛和L-色氨酸;iP7G、cZR、tZOG和tZR分别为异戊烯腺嘌呤-7-葡糖、苷顺式-玉米素-D-核糖甙、反式-玉米素-9-Β-葡萄糖苷和玉米素核苷。
Fig. 5 Dynamic changes in hormone contents of tomato graft union GA15,GA8,GA3 and GA5 were gibberellin A15,gibberellin A8,gibberellin A3 and gibberellin A5,some GA8 data are not detected because the levels of the hormones in the samples were below the detection limit of the instrument;IAA,MEIAA,ICAld and TRP were indole-3-acetic acid,methyl indole-3-acetate,indole-3-carboxaldehyde and L-tryptophan;iP7G,cZR,tZOG were N6-Isopentenyl-adenine-7-glucoside,cis-Zeatin riboside,trans-zeatin-O-glucoside and trans-zeatin riboside,respectively.
| [1] | Asahina M, Azuma K, Pitaksaringkarn W, Yamazaki T, Mitsuda N, Ohme-Takagi M, Yamaguchi S, Kamiya Y, Okada K, Nishimura T, Koshiba T, Yokota T, Kamada H, Satoh S. 2011. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 108 (38):16128-16132. |
| [2] |
Asahina M, Iwai H, Kikuchi A, Yamaguchi S, Kamiya Y, Kamada H, Satoh S. 2002. Gibberellin produced in the cotyledon is required for cell division during tissue reunion in the cortex of cut cucumber and tomato hypocotyls. Plant Physiology, 129:201-210.
doi: 10.1104/pp.010886 URL |
| [3] |
Ahmad I, Kamran M, Meng X P, Ali S, Ahmad S, Gao Z Q, Liu T N, Han Q F. 2021. Hormonal changes with uniconazole trigger canopy apparent photosynthesis and grain filling in wheat crop in a semi-arid climate. Protoplasma, 258:139-150.
doi: 10.1007/s00709-020-01559-0 URL |
| [4] | Bai Longqiang, Liu Yumei, Mu Ying, He Chaoxing, Xie Bingyan, Yu Xianchang, Li Yansu. 2018. Effects of gibberellin on nitrogen metabolism and uptake of cucumber under suboptimal root-zone temperature. Acta Horticulturae Sinica, 45 (10):1917-1928. (in Chinese) |
| 白龙强, 刘玉梅, 慕英, 贺超兴, 闫妍, 谢丙炎, 于贤昌, 李衍素. 2018. 赤霉素对根区亚低温下黄瓜幼苗氮代谢与吸收的影响. 园艺学报, 45 (10):1917-1928. | |
| [5] | Biemelt S, Tschiersch H, Sonnewald U. 2004. Impact of altered gibberellin metabolism on biomass accumulation,lignin biosynthesis,and photosynthesis in transgenic tobacco plants. Plant Physioloy, 135:254-265. |
| [6] |
Cantero-Navarro E, Romero-Aranda R, Fernández-Muñoz R, Martínez-Andújar C, Pérez-Alfocea F, Albacete A. 2016. Improving agronomic water use efficiency in tomato by rootstock-mediated hormonal regulation of leaf biomass. Plant Science, 251:90-100.
doi: S0168-9452(16)30032-2 pmid: 27593467 |
| [7] |
Chapman E J, Greenham K, Castillejo C, Sartor R, Bialy A, Sun T P, Estelle M. 2012. Hypocotyl transcriptome reveals auxin regulation of growth-promoting genes through GA-dependent and -independent pathways. PLoS ONE, 7 (5):e36210.
doi: 10.1371/journal.pone.0036210 URL |
| [8] |
Cui Q Q, Xie L L, Dong C J, Gao L H, Shang Q M. 2021. Stage-specific events in tomato graft formation and the regulatory effects of auxin and cytokinin. Plant Science, 304:110803.
doi: 10.1016/j.plantsci.2020.110803 URL |
| [9] |
Dayan J, Voronin N, Gong F, Sun T P, Hedden P, Fromm H, Aloni R. 2012. Leaf-induced gibberellin signaling is essential for internode elongation,cambial activity,and fiber differentiation in tobacco stems. The Plant Cell, 24 (1):66-79.
doi: 10.1105/tpc.111.093096 URL |
| [10] |
del Pozo J C, Lopez-Matas M A, Ramirez-Parra E, Gutierrez C. 2005. Hormonal control of the plant cell cycle. Physiologia Plantarum, 123 (2):173-183.
doi: 10.1111/j.1399-3054.2004.00420.x URL |
| [11] |
Estañ M T, Martinez-Rodriguez M M, Perez-Alfocea F, Flowers T J, Bolarin M C. 2005. Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot. Journal of Experimental Botany, 56 (412):703-712.
doi: 10.1093/jxb/eri027 URL |
| [12] | Fletcher R A, Hofstra G, Gao J G. 1986. Comparative fungitoxic and plant growth regulating properties of triazole derivatives. Plant Cell Physiology, 27 (2):367-371. |
| [13] |
Frigerio M, Alabadí D, Pérez-Gómez J, García-Cárcel L, Phillips A L, Hedden P, Blázquez M A. 2006. Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiology, 142 (2):553-563.
doi: 10.1104/pp.106.084871 pmid: 16905669 |
| [14] | Goldschmidt E E. 2014. Plant grafting:new mechanisms,evolutionary implications. Frontiers in Plant Science, 5 (727):727. |
| [15] | Han Min, Cao Bili, Liu Shusen, Xu Kun. 2018. Effects of rootstock and scion interaction on chilling tolerance of grafted tomato seedlings. Acta Horticulturae Sinica, 45 (2):279-288. (in Chinese) |
| 韩敏, 曹逼力, 刘树森, 徐坤. 2018. 番茄嫁接苗根穗互作对其耐冷性的影响. 园艺学报, 45 (2):279-288. | |
| [16] |
Hedden P. 2020. The current status of research on gibberellin biosynthesis. Plant Cell Physiology, 61 (11):1832-1849.
doi: 10.1093/pcp/pcaa092 URL |
| [17] |
Inzé D, de Veylder L. 2006. Cell cycle regulation in plant development. Annual Review of Genetics, 40:77-105.
doi: 10.1146/annurev.genet.40.110405.090431 URL |
| [18] | Iwase A, Mitsuda N, Ikeuchi M, Ohnuma M, Koizuka C, Kawamoto K, Imamura J, Ezura H, Sugimoto K. 2013. Arabidopsis WIND 1 induces callus formation in rapeseed,tomato,and tobacco. Plant Signaling & Behavior, 8 (12):e27432. |
| [19] |
Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, Inoue Y, Seki M, Sakakibara H, Sugimoto K, Ohme-Takagi M. 2011. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Current Biology, 21:508-514.
doi: 10.1016/j.cub.2011.02.020 URL |
| [20] | Izumi K, Nakagawa S, Kobayashi M, Oshio H, Sakurai A, Takahashi N. 1988. Levels of IAA,cytokinins,ABA and ethylene in rice plants as affected by a gibberellin biosynthesis inhibitor,uniconazole-P. Plant and Cell Physiology, 29 (1):97-104. |
| [21] | Kieber J J, Schaller G E. 2018. Cytokinin signaling in plant development. Development, 145 (4):dev149344. |
| [22] |
Kunwar S, Paret M L, Olson S M, Ritchie L, Rich J R, Freeman J, McAvoy T. 2015. Grafting using rootstocks with resistance to Ralstonia solanacearum against Meloidogyne incognita in tomato production. Plant Disease, 99:119-124.
doi: 10.1094/PDIS-09-13-0936-RE pmid: 30699747 |
| [23] |
Lee J M, Kubota C, Tsao S J, Bie Z L, Echevarria P H, Morra L, Oda M. 2010. Current status of vegetable grafting:diffusion,grafting techniques,automation. Scientia Horticulturae, 127 (2):93-105.
doi: 10.1016/j.scienta.2010.08.003 URL |
| [24] | Lee J M, Oda M. 2003. Grafting of herbaceous vegetable and ornamental crops. Horticultural Reviews, 28:61-124. |
| [25] |
Lee K M, Lim C S, Muneer S, Jeong B R. 2016. Functional vascular connections and light quality effects on tomato grafted unions. Scientia Horticulturae, 201:306-317.
doi: 10.1016/j.scienta.2016.02.013 URL |
| [26] |
León J, Rojo E, Sánchez-Serrano J J. 2001. Wound signalling in plants. Journal of Experimental Botany, 52 (354):1-9.
pmid: 11181708 |
| [27] | Li Maofu, Yang Yuan, Wang Hua, Liu Jiashen, Jin Wanmei. 2019. Effects of gibberellin on growth and development of rose‘Carola’. Acta Horticulturae Sinica, 46 (4):749-760. (in Chinese) |
| 李茂福, 杨媛, 王华, 刘佳棽, 金万梅. 2019. 赤霉素对露地栽培月季‘卡罗拉’生长发育的影响. 园艺学报, 46 (4):749-760. | |
| [28] |
Lin C H, Hsu S T, Tzeng K C, Wang J F. 2008. Application of a preliminary screen to select locally adapted resistant rootstock and soil amendment for integrated management of tomato bacterial wilt in Taiwan. Plant Disease, 92:909-916.
doi: 10.1094/PDIS-92-6-0909 URL |
| [29] |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods, 25 (4):402-408.
doi: 10.1006/meth.2001.1262 pmid: 11846609 |
| [30] |
Lu S F, Song Y R. 1999. Relation between phytohormone level and vascular bridge differentiation in graft union of explanted internode autografting. Chinese Science Bulletin, 44 (20):1874-1878.
doi: 10.1007/BF02886344 URL |
| [31] | Magome H, Nomura T, Hanada A, Takeda-Kamiya N, Ohnishi T, Shinma Y, Katsumata T, Kawaide H, Kamiya Y, Yamaguchi S. 2013. CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice. Proceedings of the National Academy of Sciences of the United States of America, 110 (5):1947-1952. |
| [32] |
Martínez-Andújar C, Ruiz-Lozano J M, Dodd I C, Albacete A, Pérez-Alfocea F. 2017. Hormonal and nutritional features in contrasting rootstock-mediated tomato growth under low-phosphorus nutrition. Frontiers in Plant Science, 8:533.
doi: 10.3389/fpls.2017.00533 pmid: 28443121 |
| [33] | Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Václavíková K, Miyawaki K, Kakimoto T. 2008. Cytokinins are central regulators of cambial activity. Proceedings of the National Academy of Sciences of the United States of America, 105 (50):20027-20031. |
| [34] |
Matsuoka K, Sugawara E, Aoki R, Takuma K, Terao-Morita M, Satoh S, Asahina M. 2016. Differential cellular control by cotyledon-derived phytohormones involved in graft reunion of Arabidopsis hypocotyls. Plant Cell Physiology, 57 (12):2620-2631.
doi: 10.1093/pcp/pcw177 URL |
| [35] | Melnyk C W, Gabel A, Hardcastle T J, Robinson S, Miyashima S, Grosse I, Meyerowitz E M. 2018. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proceedings of the National Academy of Sciences of the United States of America, 115 (10):E2447-E2456. |
| [36] |
Melnyk C W, Schuster C, Leyser O, Meyerowitz E M. 2015. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Current Biology, 25 (10):1306-1318.
doi: 10.1016/j.cub.2015.03.032 URL |
| [37] |
Moreno M M, Villena J, González-Mora S, Moreno C. 2019. Response of healthy local tomato(Solanum lycopersicum L.)populations to grafting in organic farming. Scientific Reports, 9 (1):4592.
doi: 10.1038/s41598-019-41018-2 pmid: 30872790 |
| [38] |
Mun B, Jang Y, Goto E, Ishigami Y, Chun C. 2011. Measurement system of whole-canopy carbon dioxide exchange rates in grafted cucumber transplants in which scions were exposed to different water regimes using a semi-open multi-chamber. Scientia Horticulturae, 130 (3):607-614.
doi: 10.1016/j.scienta.2011.08.017 URL |
| [39] |
Nomura T, Magome H, Hanada A, Takeda-Kamiya N, Mander L N, Kamiya Y, Yamaguchi S. 2013. Functional analysis of Arabidopsis CYP714A1 and CYP714A 2 reveals that they are distinct gibberellin modification enzymes. Plant Cell Physiology, 54 (11):1837-1851.
doi: 10.1093/pcp/pct125 URL |
| [40] |
Notaguchi M, Kurotani K, Sato Y, Tabata R, Kawakatsu Y, Okayasu K, Sawai Y, Okada R, Asahina M, Ichihashi Y, Shirasu K, Suzuki T, Niwa M, Higashiyama T. 2020. Cell-cell adhesion in plant grafting is facilitated by β-1,4-glucanases. Science, 369:698-702.
doi: 10.1126/science.abc3710 URL |
| [41] |
Ntatsi G, Savvas D, Papasotiropoulos V, Katsileros A, Zrenner R M, Hincha D K, Zuther E, Schwarz D. 2017. Rootstock sub-optimal temperature tolerance determines transcriptomic responses after long-term root cooling in rootstocks and scions of grafted tomato plants. Frontiers in Plant Science, 8:911.
doi: 10.3389/fpls.2017.00911 URL |
| [42] |
Oda M, Maruyama M, Mori G. 2005. Water transfer at graft union of tomato plants grafted onto Solanum rootstocks. Journal of the Japanese Society for Horticultural Science, 74 (6):458-463.
doi: 10.2503/jjshs.74.458 URL |
| [43] | Ozturk A, Serdar U, Balci G. 2009. The influence of different nursery conditions on graft success and plant survival using the inverted radicle grafting method on the chestnut. Acta Horticulturae, 815:193-198. |
| [44] | Pang Shichan, Guo Shuang, Ren Kuiyu, Wang Shuaishuai, Yang Shangdong. 2020. Impact of grafting on soil microbial properties and bacterial community structure in tomato rhizosphere. Acta Horticulturae Sinica, 47 (2):253-263. (in Chinese) |
| 庞师婵, 郭霜, 任奎瑜, 王帅帅, 杨尚东. 2020. 番茄/茄子嫁接对其根际土壤生物学性状及细菌群落结构的影响. 园艺学报, 47 (2):253-263. | |
| [45] |
Pitaksaringkarn W, Ishiguro S, Asahina M, Satoh S. 2014. ARF6 and ARF8 contribute to tissue reunion in incised Arabidopsis inflorescence stems. Plant Biotechnology, 31 (1):49-53.
doi: 10.5511/plantbiotechnology.13.1028b URL |
| [46] |
Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke C S. 2011. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. The Plant Cell, 23:1322-1336.
doi: 10.1105/tpc.111.084020 URL |
| [47] |
Riga P, Benedicto L, García-Flores L, Villaño D, Medina S, Gil-Izquierdo Á. 2016. Rootstock effect on serotonin and nutritional quality of tomatoes produced under low temperature and light conditions. Journal of Food Composition and Analysis, 46:50-59.
doi: 10.1016/j.jfca.2015.11.003 URL |
| [48] |
Rivero R M, Ruiz J M, Sánchez E, Romero L. 2003. Does grafting provide tomato plants an advantage against H2O2 production under conditions of thermal shock? Physiologia Plantarum, 117:44-50.
doi: 10.1034/j.1399-3054.2003.1170105.x URL |
| [49] |
Sánchez-Rodríguez E, Ruiz J M, Ferreres F, Moreno D A. 2011. Phenolic metabolism in grafted versus nongrafted cherry tomatoes under the influence of water stress. Journal of Agricultural and Food Chemistry, 59 (16):8839-8846.
doi: 10.1021/jf201754t pmid: 21732696 |
| [50] |
Sasaki E, Ogura T, Takei K, Kojima M, Kitahata N, Sakakibara H, Asami T, Shimada Y. 2013. Uniconazole,a cytochrome P450 inhibitor,inhibits trans-zeatin biosynthesis in Arabidopsis. Phytochemistry, 87:30-38.
doi: 10.1016/j.phytochem.2012.11.023 pmid: 23280040 |
| [51] |
Shibuya T, Itagaki K, Wang Y, Endo R. 2015. Grafting transiently suppresses development of powdery mildew colonies,probably through a quantitative change in water relations of the host cucumber scions during graft healing. Scientia Horticulturae, 192:197-199.
doi: 10.1016/j.scienta.2015.06.010 URL |
| [52] | Vu N T, Xu Z H, Kim Y S, Kang H M, Kim I S. 2014. Effect of nursery environmental condition and different cultivars on survival rate of grafted tomato seedling. Acta Horticulturae, 1037:765-770. |
| [53] |
Wei Y Z, Dong C, Zhang H N, Zheng X W, Shu B, Shi S Y, Li W C. 2017. Transcriptional changes in litchi(Litchi chinensis Sonn.)inflorescences treated with uniconazole. PLoS ONE, 12 (4):e0176053.
doi: 10.1371/journal.pone.0176053 URL |
| [54] |
Xie L L, Dong C J, Shang Q M. 2019. Gene co-expression network analysis reveals pathways associated with graft healing by asymmetric profiling in tomato. BMC Plant Biology, 19 (1):373.
doi: 10.1186/s12870-019-1976-7 URL |
| [55] |
Yin H, Yan B, Sun J, Jia P F, Zhang Z J, Yan X S, Chai J, Ren Z Z, Zheng G C, Liu H. 2012. Graft-union development:a delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. Journal of Experimental Botany, 63 (11):4219-4232.
doi: 10.1093/jxb/ers109 URL |
| [56] |
Zhai L M, Wang X M, Tang D, Qi Q, Yer H, Jiang X N, Han Z H, McAvoy R, Li W, Li Y. 2021. Molecular and physiological characterization of the effects of auxin-enriched rootstock on grafting. Horticulture Research, 8:74.
doi: 10.1038/s41438-021-00509-y URL |
| [57] |
Zhang K, Letham D S, John P C L. 1996. Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H 1 histone kinase. Planta, 200 (1):2-12.
pmid: 8987615 |
| [58] |
Zhang M C, Duan L S, Tian X L, He Z P, Li J M, Wang B M, Li Z H. 2007. Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis,hormones and antioxidant system. Journal of Plant Physiology, 164 (6):709-717.
doi: 10.1016/j.jplph.2006.04.008 URL |
| [59] |
Zhang Z H, Cao B L, Gao S, Xu K. 2019. Grafting improves tomato drought tolerance through enhancing photosynthetic capacity and reducing ROS accumulation. Protoplasma, 256:1013-1024.
doi: 10.1007/s00709-019-01357-3 URL |
| [1] | 史洪丽, 李腊, 郭翠梅, 余婷婷, 简伟, 杨星勇. 番茄灰霉病生防菌株TL1的分离、鉴定及其生防能力分析[J]. 园艺学报, 2023, 50(1): 79-90. |
| [2] | 忽靖宇, 阙开娟, 缪田丽, 吴少政, 王田田, 张磊, 董鲜, 季鹏章, 董家红. 侵染鸢尾的番茄斑萎病毒鉴定[J]. 园艺学报, 2023, 50(1): 170-176. |
| [3] | 郑积荣, 王同林, 胡松申. 高品质番茄新品种‘杭杂603’[J]. 园艺学报, 2022, 49(S2): 103-104. |
| [4] | 郑积荣, 王同林. 番茄新品种‘杭杂601’[J]. 园艺学报, 2022, 49(S2): 105-106. |
| [5] | 郑积荣, 王同林. 樱桃番茄新品种‘杭杂503’[J]. 园艺学报, 2022, 49(S2): 107-108. |
| [6] | 黄婷婷, 刘淑芹, 张永志, 李 平, 张志焕, 宋立波. 樱桃番茄新品种‘樱莎红4号’[J]. 园艺学报, 2022, 49(S2): 109-110. |
| [7] | 张前荣, 李大忠, 裘波音, 林 珲, 马慧斐, 叶新如, 刘建汀, 朱海生, 温庆放. 设施番茄新品种‘闽农科2号’[J]. 园艺学报, 2022, 49(S1): 73-74. |
| [8] | 韩帅, 吴婕, 张河庆, 席亚东. 四川莴笋上番茄斑萎病毒的电镜观察与小RNA测序鉴定[J]. 园艺学报, 2022, 49(9): 2007-2016. |
| [9] | 陈礼浪, 杨天章, 蔡儒平, 林小漫, 邓南康, 车海彦, 林雅婷, 孔祥义. 海南西番莲主要病毒种类的分子检测与鉴定[J]. 园艺学报, 2022, 49(8): 1785-1794. |
| [10] | 路涛, 余宏军, 李强, 蒋卫杰. 叶果量调控对番茄生长发育、果实品质和产量的影响[J]. 园艺学报, 2022, 49(6): 1261-1274. |
| [11] | 祁利潘, 李越, 王磊, 冯琰, 王宽, 尹江, 郭华春. 马铃薯与枸杞嫁接愈合过程的解剖学观察[J]. 园艺学报, 2022, 49(4): 868-874. |
| [12] | 崔东禹, 李长青, 孙焱鑫, 王激清, 邹国元, 杨俊刚. 温室番茄东西向栽培条件下矮化密植对其生长和产量的影响[J]. 园艺学报, 2022, 49(4): 875-884. |
| [13] | 陈同强, 张天柱, 王晓卓. 光照对番茄果实中番茄红素生物合成的调控研究进展[J]. 园艺学报, 2022, 49(4): 907-923. |
| [14] | 彭轶, 李元慧, 杨瑞, 张子怡, 李亚楠, 韩云昊, 赵文超, 王绍辉. 茉莉酸合成基因LoxD参与调控番茄的抗旱性[J]. 园艺学报, 2022, 49(2): 319-331. |
| [15] | 王晋, 王新宇, 沈渊博, 张清花, 娄茜棋, 张世杰, 赵攀, 梁燕. 番茄果实叶绿体发育调控及其应用的研究进展[J]. 园艺学报, 2022, 49(12): 2669-2682. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司