
园艺学报 ›› 2022, Vol. 49 ›› Issue (2): 265-280.doi: 10.16420/j.issn.0513-353x.2020-1000
黎春红1,2, 汪开拓1,2,*( ), 雷长毅1, 许凤3, 季娜娜2, 蒋永波1
), 雷长毅1, 许凤3, 季娜娜2, 蒋永波1
收稿日期:2021-10-30
修回日期:2022-01-18
出版日期:2022-02-25
发布日期:2022-02-28
通讯作者:
汪开拓
E-mail:wangkaituo83@gmail.com
基金资助:
LI Chunhong1,2, WANG Kaituo1,2,*( ), LEI Changyi1, XU Feng3, JI Nana2, JIANG Yongbo1
), LEI Changyi1, XU Feng3, JI Nana2, JIANG Yongbo1
Received:2021-10-30
Revised:2022-01-18
Online:2022-02-25
Published:2022-02-28
Contact:
WANG Kaituo
E-mail:wangkaituo83@gmail.com
摘要:
以高度保守的BRLZ(PF07716)和DOG1(PF14144)结构域为种子序列,通过生物信息分析共鉴定得到15个桃TGA(TGACG motif-binding factor)家族基因,这些基因分布于桃的4条染色体上,其编码蛋白大小介于333 ~ 546 aa,分子量介于37.07 ~ 61.47 kD,等电点介于6.01 ~ 8.59,均定位于细胞核上。根据系统进化关系,拟南芥、大豆、番茄、水稻和桃的 TGA 家族分为5 个亚族,其中桃 TGA 家族成员主要分布于第Ⅰ、Ⅱ和Ⅳ亚族。对桃TGA家族成员启动子区域的顺式调控元件进行预测分析,其启动子区含有至少1个激素或逆境胁迫响应元件。经RNA-seq 数据分析可知,β-氨基丁酸(β- aminobutyric acid,BABA)处理和匍枝根霉(Rhizopus stolonifer)侵染能诱导桃TGA成员表达,其中PpTGA1-1在处理后12 h内上调最为显著;显著表达的PpTGA1-1通过与PpNPR1蛋白相互作用赋予PpNPR1蛋白DNA结合功能,启动一系列病程相关(pathogenesis-related,PR)基因的表达,从而诱导果实敏化(priming)抗性。桃 TGA 家族成员(尤其PpTGA1-1)可直接响应激发子诱导和病原菌侵染,并通过修饰PpNPR1蛋白从而在防卫反应中发挥重要调控作用。
中图分类号:
黎春红, 汪开拓, 雷长毅, 许凤, 季娜娜, 蒋永波. 桃TGA家族鉴定及BABA诱导的抗病表达分析[J]. 园艺学报, 2022, 49(2): 265-280.
LI Chunhong, WANG Kaituo, LEI Changyi, XU Feng, JI Nana, JIANG Yongbo. Identification of TGA Gene Family in Peach and Analysis of Expression Mode Involved in a BABA-Induced Disease Resistance[J]. Acta Horticulturae Sinica, 2022, 49(2): 265-280.
| 基因(ID) Gene/vector | 引物序列(5′-3′) Primer sequences |
|---|---|
| Pp18S rRNA(L28749.1) PpNPR1(LOC18771764) PpPR1(LOC18789999) PpPR2(LOC109950426) PpPR5(LOC18791880) PpTGA1-1(XM_007205344.2) PpTGA1-2(XM_020566027.1) PpTGA2.2(XM_007218305.2) PpTGA7-1(XM_020555774.1) PpTGA7-2(XM_020555775.1) PpTGA7-3(XM_007222027.2) PpTGA9-1(XM_007204982.2) PpTGA9-2(XM_020567215.1) PpTGA9-3(XM_020567216.1) PpTGA10-1(XM_007204506.2) PpTGA10-2(XM_020567712.1) PpTGA10-3(XM_020567713.1) PpHBP-1b-1(XM_020568216.1) PpHBP-1b-2(XM_007226654.2) PpHBP-1b-3(XM_020558260.1) | F:ATGGCCGTTCTTAGTTGGTG;R:GTACAAAGGGCAGGGACGTA F:CGGCAAAGCGTGTGAGAGAT;R:CTGTCCAAGCCAAGTGCCAA F:CCGGTCAGCCACCAAAATGA;R:GCCTCAAAGCGCAGTCGTAT F:CCGGAAGGGCCATAGAAACC;R:TCGGCTGTTTGCTTGGTGAA F:CGGAGTTCACGACAGGTTCG;R:ATTCAAGTCGGCCACGCATC F:CTCATGGAATCCTGGGGCCT;R:CTTTTACGAGCAGCCTCCCG F:TGCCGCAAAAGCTGACGTAT;R:TCTCTGAAGGGCGAAACCCT F:TGTGGCTTGGTGGTTTTCGT;R:CCTGTTGGGAGGATTGCTGC F:CACTGGAGACCTTTGTGGGC;R:CACGCAGCCGGTGAAAGTAT F:CGGAAGATGCTCTCACGCAG;R:CCCACAAAGGTCTCCAGTGC F:AGCTGCTCGCAAAAGTCGTAT;R:AGGCACCCTGTTTTCTAGCTCT F:TTTCTGCCACATGTCCCGATT;R:CAACTCTATGACTCGCCATGCAA F:CTGAGCGTTGCTTCCTCTGG;R:CGAGGACTGCTGGAGGCTAT F:GCAGGCTCAAGACATGGCAATA;R:ACGTCTCAATGTCTTTTCCTGGG F:AGCCTATGCACGTAGAGCCA;R:AGCATCTTTGCGAGGGTTGG F:GAACTTCATCAAAGACAGTGGAGC;R:TGTGATGGTCTTGTGACTGTCC F:CCAACCCTCGCAAAGATGCT;R:GGGTCTGGTGTTTTGGGTCC F:TCAAAATCGTGAAGCCGCCA;R:CACGCTGAAGCTCTTGCTCA F:TCCTTCTATTTCAGAGGAGACGACA;R:CGTGAGGGAAGACAGTAGCG F:CCTTGAACACGAGCACTGGG;R:AAAGTGTCCGTTTCCCGACG |
表1 本试验中测定基因的特异性引物序列
Table 1 The gene-specific primer sequences used in this experiment
| 基因(ID) Gene/vector | 引物序列(5′-3′) Primer sequences |
|---|---|
| Pp18S rRNA(L28749.1) PpNPR1(LOC18771764) PpPR1(LOC18789999) PpPR2(LOC109950426) PpPR5(LOC18791880) PpTGA1-1(XM_007205344.2) PpTGA1-2(XM_020566027.1) PpTGA2.2(XM_007218305.2) PpTGA7-1(XM_020555774.1) PpTGA7-2(XM_020555775.1) PpTGA7-3(XM_007222027.2) PpTGA9-1(XM_007204982.2) PpTGA9-2(XM_020567215.1) PpTGA9-3(XM_020567216.1) PpTGA10-1(XM_007204506.2) PpTGA10-2(XM_020567712.1) PpTGA10-3(XM_020567713.1) PpHBP-1b-1(XM_020568216.1) PpHBP-1b-2(XM_007226654.2) PpHBP-1b-3(XM_020558260.1) | F:ATGGCCGTTCTTAGTTGGTG;R:GTACAAAGGGCAGGGACGTA F:CGGCAAAGCGTGTGAGAGAT;R:CTGTCCAAGCCAAGTGCCAA F:CCGGTCAGCCACCAAAATGA;R:GCCTCAAAGCGCAGTCGTAT F:CCGGAAGGGCCATAGAAACC;R:TCGGCTGTTTGCTTGGTGAA F:CGGAGTTCACGACAGGTTCG;R:ATTCAAGTCGGCCACGCATC F:CTCATGGAATCCTGGGGCCT;R:CTTTTACGAGCAGCCTCCCG F:TGCCGCAAAAGCTGACGTAT;R:TCTCTGAAGGGCGAAACCCT F:TGTGGCTTGGTGGTTTTCGT;R:CCTGTTGGGAGGATTGCTGC F:CACTGGAGACCTTTGTGGGC;R:CACGCAGCCGGTGAAAGTAT F:CGGAAGATGCTCTCACGCAG;R:CCCACAAAGGTCTCCAGTGC F:AGCTGCTCGCAAAAGTCGTAT;R:AGGCACCCTGTTTTCTAGCTCT F:TTTCTGCCACATGTCCCGATT;R:CAACTCTATGACTCGCCATGCAA F:CTGAGCGTTGCTTCCTCTGG;R:CGAGGACTGCTGGAGGCTAT F:GCAGGCTCAAGACATGGCAATA;R:ACGTCTCAATGTCTTTTCCTGGG F:AGCCTATGCACGTAGAGCCA;R:AGCATCTTTGCGAGGGTTGG F:GAACTTCATCAAAGACAGTGGAGC;R:TGTGATGGTCTTGTGACTGTCC F:CCAACCCTCGCAAAGATGCT;R:GGGTCTGGTGTTTTGGGTCC F:TCAAAATCGTGAAGCCGCCA;R:CACGCTGAAGCTCTTGCTCA F:TCCTTCTATTTCAGAGGAGACGACA;R:CGTGAGGGAAGACAGTAGCG F:CCTTGAACACGAGCACTGGG;R:AAAGTGTCCGTTTCCCGACG |
| 所用载体 Vector | 引物名称 Primer name | 寡核苷酸引物对 Oligonucleotide primers |
|---|---|---|
| pGADT7 | PpNPR1-SmaI-F PpNPR1-BamHI-R | GGCCAGTGAATTCCACCCGGGCCCGGGATGGAATTCAAAGCCGGAGTC CAGCTCGAGCTCGATGGATCCGGATCCTTGATTGAGGGTGAGCATTCCA |
| pGBKT7 | PpTGA1-1-SmaI-F PpTGA1-1-BamHI-R | CATGGAGGCCGAATTCCCGGGATGAATTCTCCATCCACCCAGTT ATGCGGCCGCTGCAGGTCGACGGCAGGCTCACGAGGACG |
表2 酵母双杂实验所用引物
Table 2 The primer sequences used in yeast two-hybrid
| 所用载体 Vector | 引物名称 Primer name | 寡核苷酸引物对 Oligonucleotide primers |
|---|---|---|
| pGADT7 | PpNPR1-SmaI-F PpNPR1-BamHI-R | GGCCAGTGAATTCCACCCGGGCCCGGGATGGAATTCAAAGCCGGAGTC CAGCTCGAGCTCGATGGATCCGGATCCTTGATTGAGGGTGAGCATTCCA |
| pGBKT7 | PpTGA1-1-SmaI-F PpTGA1-1-BamHI-R | CATGGAGGCCGAATTCCCGGGATGAATTCTCCATCCACCCAGTT ATGCGGCCGCTGCAGGTCGACGGCAGGCTCACGAGGACG |
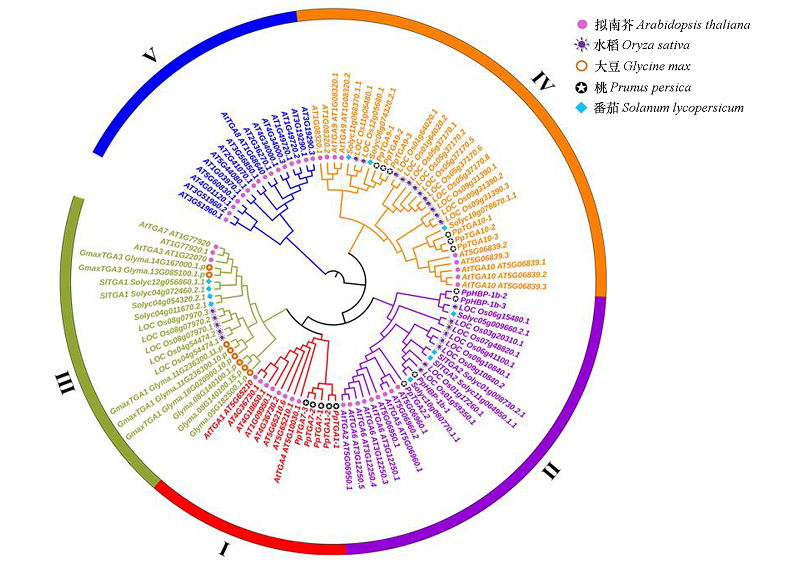
图2 桃和模式植物拟南芥、大豆、番茄及水稻 TGA家族的系统进化树
Fig. 2 The phylogenetic classification of TGA family in Prunus persica,Arabidopsis thaliana,Glycine max, Solanum lycopersicum and Oryza sativa
| 基因名称 Gene name | 蛋白ID(NCBI) Protein ID | 编码序列长度/bp CDS length | 蛋白特性 Protein property | ||||
|---|---|---|---|---|---|---|---|
| 氨基酸数 Number of amino acids | 分子量/kD MW | 等电点 pI | 不稳定系数 Instability index | 平均亲水性 GRAVY | |||
| PpTGA1-1 PpTGA1-2 PpTGA2.2 PpTGA7-1 PpTGA7-2 PpTGA7-3 PpTGA9-1 PpTGA9-2 PpTGA9-3 PpTGA10-1 PpTGA10-2 PpTGA10-3 PpHBP-1b-1 PpHBP-1b-2 PpHBP-1b-3 | XP_007205406.1 XP_020421616.1 XP_007218367.2 XP_020411363.1 XP_020411364.1 XP_007222089.2 XP_007205044.2 XP_020422804.1 XP_020422805.1 XP_007204568.1 XP_020423301.1 XP_020423302.1 XP_020423805.1 XP_007226716.1 XP_020413849.1 | 1 092 1 089 1 002 1 131 1 116 1 104 1 596 1 515 1 467 1 641 1 638 1 641 1 359 1 395 1 221 | 363 362 333 376 371 367 531 504 488 546 545 546 452 464 406 | 40.99 40.86 37.07 42.78 42.12 41.67 58.59 55.55 53.87 61.47 61.39 61.47 49.95 51.67 45.14 | 6.41 6.41 8.59 6.51 6.51 6.37 6.45 6.58 6.32 6.64 6.64 6.64 7.84 6.01 6.38 | 49.13 48.70 58.49 52.03 51.20 50.29 56.81 56.70 56.54 67.80 67.75 67.80 62.22 40.31 41.33 | -0.468 -0.459 -0.570 -0.594 -0.587 -0.584 -0.538 -0.555 -0.520 -0.757 -0.757 -0.757 -0.616 -0.539 -0.509 |
表3 桃 TGA家族成员的理化性质
Table 3 Characterisation of the physical and chemical properties of TGA family members in peach
| 基因名称 Gene name | 蛋白ID(NCBI) Protein ID | 编码序列长度/bp CDS length | 蛋白特性 Protein property | ||||
|---|---|---|---|---|---|---|---|
| 氨基酸数 Number of amino acids | 分子量/kD MW | 等电点 pI | 不稳定系数 Instability index | 平均亲水性 GRAVY | |||
| PpTGA1-1 PpTGA1-2 PpTGA2.2 PpTGA7-1 PpTGA7-2 PpTGA7-3 PpTGA9-1 PpTGA9-2 PpTGA9-3 PpTGA10-1 PpTGA10-2 PpTGA10-3 PpHBP-1b-1 PpHBP-1b-2 PpHBP-1b-3 | XP_007205406.1 XP_020421616.1 XP_007218367.2 XP_020411363.1 XP_020411364.1 XP_007222089.2 XP_007205044.2 XP_020422804.1 XP_020422805.1 XP_007204568.1 XP_020423301.1 XP_020423302.1 XP_020423805.1 XP_007226716.1 XP_020413849.1 | 1 092 1 089 1 002 1 131 1 116 1 104 1 596 1 515 1 467 1 641 1 638 1 641 1 359 1 395 1 221 | 363 362 333 376 371 367 531 504 488 546 545 546 452 464 406 | 40.99 40.86 37.07 42.78 42.12 41.67 58.59 55.55 53.87 61.47 61.39 61.47 49.95 51.67 45.14 | 6.41 6.41 8.59 6.51 6.51 6.37 6.45 6.58 6.32 6.64 6.64 6.64 7.84 6.01 6.38 | 49.13 48.70 58.49 52.03 51.20 50.29 56.81 56.70 56.54 67.80 67.75 67.80 62.22 40.31 41.33 | -0.468 -0.459 -0.570 -0.594 -0.587 -0.584 -0.538 -0.555 -0.520 -0.757 -0.757 -0.757 -0.616 -0.539 -0.509 |
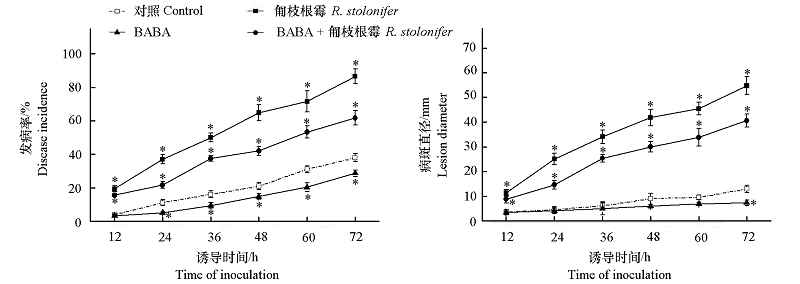
图6 BABA诱导和匍枝根霉侵染对桃果实采后发病率及病斑直径的影响
Fig. 6 Effect of BABA elicitation and Rhizopus stolonifer infection on disease incidence and lesion diameter in peach *P < 0.05.
| [1] |
Buscaill P, Rivas S. 2014. Transcriptional control of plant defence responses. Current Opinion in Plant Biology, 20 (1):35-46.
doi: 10.1016/j.pbi.2014.04.004 URL |
| [2] | Chen Si, Wang Li, Xia Ming-xing, Wu Dong-zhi, Liao Yun-xia, Wang Kai-tuo, Zheng Yong-hua. 2019. Effects of BABA treatment on redox status and its impact on induction of disease resistance in postharvest peaches. Food Science, 40 (1):217-223. (in Chinese) |
| 陈偲, 汪立, 夏明星, 伍冬志, 廖云霞, 汪开拓, 郑永华. 2019. β-氨基丁酸处理对采后桃果实还原势的影响及抗病性的诱导作用. 食品科学, 40 (1):217-223. | |
| [3] |
Chisholm S, Coaker G, Day B, Staskawicz B. 2006. Host-microbe interactions:shaping the evolution of the plant immune response. Cell, 124 (4):803-814.
doi: 10.1016/j.cell.2006.02.008 pmid: 16497589 |
| [4] |
Conrath U, Beckers G J M, Langenbach C J G, Jaskiewicz M R. 2015. Priming for enhanced defense. Annual Review of Phytopathology, 53 (1):97-119.
doi: 10.1146/phyto.2015.53.issue-1 URL |
| [5] |
Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert P R. 2003. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. The Plant Cell, 15 (9):2181-2191.
doi: 10.1105/tpc.012849 URL |
| [6] | Fan Meili, Pan Lei, Zeng Wenfang, Lu Zhenhua, Cui Guochao, Meng Junren, Jin Zhe, Wang Zhiqiang, Niu Liang. 2020. Genome wide identification of MRLK family genes and expression analysis in response to Aphid infection in peach. Acta Horticulturae Sinica, 47 (1):1-13. (in Chinese) |
| 樊美丽, 潘磊, 曾文芳, 鲁振华, 崔国朝, 孟君仁, 靳哲, 王志强, 牛良. 2020. 桃 MRLK 家族全基因组鉴定及蚜虫侵染后的表达分析. 园艺学报, 47 (1):1-13. | |
| [7] | Finn R D, Clements J, Eddy S R. 2011. HMMER web server:interactive sequence similarity searching. Nucleic Acids Research, 39 (2):29-37. |
| [8] | Franco-Zorrilla J M, López-Vidriero I, Carrasco J L, Godoy M, Vera P, Solano R. 2014. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proceedings of the National Academy of Sciences of the United States of America, 111 (6):2367-2372. |
| [9] |
Geng X Q, Jin L, Shimada M, Kim M G, Mackey D. 2014. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta, 240 (1):1149-1165.
doi: 10.1007/s00425-014-2151-x URL |
| [10] |
Hamiduzzaman M M, Jakab G, Barnavon L, Neuhaus J M, Mauch-Mani B. 2005. β-Aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signaling. Molecular Plant-Microbe Interactions, 18 (8):819-829.
pmid: 16134894 |
| [11] |
Hussain R M F, Sheikh A H, Haider I, Quareshy M, Linthorst J M. 2018. Arabidopsis WRKY50 and TGA transcription factors synergistically activate expression of PR1. Frontiers in Plant Science, 9 (1):930-945.
doi: 10.3389/fpls.2018.00930 URL |
| [12] |
Ito T, Azumano M, Uwatoko C, Itoh K, Kuwahara J. 2009. Role of zinc finger structure in nuclear localization of transcription factor Sp1. Biochemical and Biophysical Research Communications, 380 (1):28-32.
doi: 10.1016/j.bbrc.2008.12.165 URL |
| [13] |
Jakoby M, Weisshaar B, Dröge-Laser W, Vicente-Carbajosa J, Kroj T, Parcy F. 2002. bZIP transcription factors in Arabidopsis. Trends in Plant Science, 7 (3):106-111.
pmid: 11906833 |
| [14] |
Kesarwani M, Yoo J, Dong X N. 2007. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiology, 144 (1):336-346.
pmid: 17369431 |
| [15] |
Lee S, Choi D. 2013. Comparative transcriptome analysis of pepper(Capsicum annuum)revealed common regulons in multiple stress conditions and hormone treatments. Plant Cell Reports, 32 (1):1351-1359.
doi: 10.1007/s00299-013-1447-9 URL |
| [16] |
Li C H, Wang J, Ji N N, Lei C Y, Zhou D X, Zheng Y H, Wang K T. 2020a. PpHOS1, a RING E 3 ubiquitin ligase,interacts with PpWRKY22 in the BABA-induced priming defense of peach fruit against Rhizopus stolonifer. Postharvest Biology and Technology, 159 (1):111029-111037.
doi: 10.1016/j.postharvbio.2019.111029 URL |
| [17] | Li C H, Wang K T, Lei C Y, Zheng Y H. 2020b. Translocation of PpNPR1 is required for β-aminobutyric acid-triggered resistance against Rhizopus stolonifer in peach fruit. Scientia Horticulturae, 272 (1):1-10. |
| [18] |
Li C H, Wang K T, Huang Y X, Lei C Y, Cao S F, Qiu L L, Xu F, Jiang Y B, Zou Y Y, Zheng Y H. 2021. Activation of the BABA-induced priming defence through redox homeostasis and the modules of TGA1 and MAPKK 5 in postharvest peach fruit. Molecular Plant Pathology, 22 (12):1624-1640.
doi: 10.1111/mpp.13134 URL |
| [19] |
Lindermayr C, Sell S, Müller B, Leister D, Durner J. 2010. Redox regulation of the NPR1-TGA 1 system of Arabidopsis thaliana by nitric oxide. The Plant Cell, 22 (1):2894-2907.
doi: 10.1105/tpc.109.066464 URL |
| [20] |
Luna E, Van Hulten M, Zhang Y, Berkowitz O, López A, Pétriacq P, Sellwood M A, Chen B, Burrell M, van de Meene A, Pieterse C M, Flors V, Ton J. 2014. Plant perception of β-aminobutyric acid is mediated by an aspartyl-tRNA synthetase. Nature Chemical Biology, 10 (1):450-456.
doi: 10.1038/nchembio.1520 URL |
| [21] |
Ma L, Sham Y Y, Walters K J, Towle H C. 2006. A critical role for the loop region of the basic helix-loop-helix/leucine zipper protein Mlx in DNA binding and glucose-regulated transcription. Nucleic Acids Research, 35 (1):35-44.
doi: 10.1093/nar/gkl987 URL |
| [22] |
Murmu J, Bush M J, Delong C, Li S T, Xu M L, Khan M, Malcolmson C, Fobert P R, Zachgo S, Hepworth S R. 2010. Arabidopsis basic leucine-zipper transcription factors TGA9 and TGA 10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly required for anther development. Plant Physiology, 154 (3):1492-1504.
doi: 10.1104/pp.110.159111 URL |
| [23] |
Mou Z, Fan W, Dong X. 2003. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell, 113 (7):935-944.
doi: 10.1016/S0092-8674(03)00429-X URL |
| [24] |
Panebianco S, Vitale A, Platania C, Restuccia C, Polizzi G, Cirvilleri G. 2014. Postharvest efficacy of resistance inducers for the control of green mold on important Sicilian citrus varieties. Journal of Plant Diseases and Protection, 121 (4):177-183.
doi: 10.1007/BF03356507 URL |
| [25] |
Porat R, Vinokur V, Weiss B, Cohen L, Daus A, Goldschmidt E E, Droby S. 2003. Induction of resistance to Penicillium digitatum in grapefruit by β-aminobutyric acid. European Journal of Plant Pathology, 109 (9):901-907.
doi: 10.1023/B:EJPP.0000003624.28975.45 URL |
| [26] |
Rahman I, Kode A, Biswas S K. 2006. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nature Protocols, 1 (6):3159-3165.
pmid: 17406579 |
| [27] |
Romanazzi G, Sanzani S M, Bi Y, Tian S P, Martínez P G, Alkan N. 2016. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biology and Technology, 122 (12):82-94.
doi: 10.1016/j.postharvbio.2016.08.003 URL |
| [28] |
Schwarzenbacher R E, Wardell G, Stassen J, Guest E, Zhang P J, Luna E, Ton J. 2020. The IBI 1 receptor of β-aminobutyric acid interacts with VOZ transcription factors to regulate abscisic acid signaling and callose-associated defense. Molecular Plant, 13 (10):1455-1469.
doi: 10.1016/j.molp.2020.07.010 pmid: 32717347 |
| [29] |
Shearer H L, Cheng Y T, Wang L P, Liu J M, Boyle P, Després C, Zhang Y L, Li X, Fobert P R. 2012. Arabidopsis clade I TGA transcription factors regulate plant defenses in an NPR1-independent fashion. Molecular Plant-Microbe Interactions, 25 (11):1459-1468.
doi: 10.1094/MPMI-09-11-0256 URL |
| [30] |
Siegmund T, Lehmann M. 2002. The Drosophila Pipsqueak protein defines a new family of helix-turn-helix DNA-binding proteins. Development Genes and Evolution, 212 (3):152-157.
pmid: 11976954 |
| [31] |
Sun T J, Busta L, Zhang Q, Ding P T, Jetter R, Zhang Y L. 2017. TGACG-binding factor 1(TGA1)and TGA4 regulate salicylic acid and pipecolic acid biosynthesis by modulating the expression of systemic acquired resistance deficient 1(SARD1)and calmodulin-binding protein 60g (CBP60g). New Phytologist, 217 (1):344-354.
doi: 10.1111/nph.2018.217.issue-1 URL |
| [32] |
Tian S P, Fan Q, Xu Y, Jiang A L. 2010. Effects of calcium on biocontrol activity of yeast antagonists against the postharvest fungal pathogen Rhizopus stolonifer. Plant Pathology, 51 (3):352-358.
doi: 10.1046/j.1365-3059.2002.00711.x URL |
| [33] |
Wang K T, Li C H, Lei C Y, Jiang Y B, Qiu L L, Zou X Y, Zheng Y H. 2020. β-Aminobutyric acid induces priming defence against Botrytis cinerea in grapefruit by reducing intercellular redox status that modifies posttranslation of VvNPR1 and its interaction with VvTGA1. Plant Physiology and Biochemistry, 156 (11):552-565.
doi: 10.1016/j.plaphy.2020.09.026 URL |
| [34] |
Wang K T, Liao Y X, Xiong Q, Kai J Q, Cao S F, Zheng Y H. 2016. Induction of direct or priming resistance against Botrytis cinerea in strawberries by β-aminobutyric acid and their effects on sucrose metabolism. Journal of Agricultural and Food Chemistry, 64 (1):5855-5865.
doi: 10.1021/acs.jafc.6b00947 URL |
| [35] |
Wang K T, Wu D Z, Bo Z Y, Chen S, Wang Z R, Zheng Y H, Fang Y. 2019. Regulation of redox status contributes to priming defense against Botrytis cinerea in grape berries treated with β-aminobutyric acid. Scientia Horticulturae, 244 (1):352-364.
doi: 10.1016/j.scienta.2018.09.074 URL |
| [36] |
Wang L, Jin P, Wang J, Jiang L, Shan T, Zheng Y H. 2015a. Effect of β-aminobutyric acid on cell wall modification and senescence in sweet cherry during storage at 20 ℃. Food Chemistry, 175 (1):471-477.
doi: 10.1016/j.foodchem.2014.12.011 URL |
| [37] |
Wang L P, Fobert P R. 2013. Arabidopsis clade I TGA factors regulate apoplastic defences against the bacterial pathogen Pseudomonas syringae through endoplasmic reticulum-based processes. PLoS ONE, 8 (9):e77378-e77390.
doi: 10.1371/journal.pone.0077378 URL |
| [38] | Wang Ping, Lu Shixiong, Liang Guoping, Ma Zonghuan, Li Wenfang, Mao Juan, Chen Baihong. 2019. Bioinformatics identification and expression analysis of Trihelix transcription factor family in apple. Acta Horticulturae Sinica, 46 (11):2082-2098. (in Chinese) |
| 王萍, 卢世雄, 梁国平, 马宗桓, 李文芳, 毛娟, 陈佰鸿. 2019. 苹果 Trihelix 转录因子家族生物信息学鉴定与基因表达分析. 园艺学报, 46 (11):2082-2098. | |
| [39] |
Wang Z H, Cheng K, Wan L Y, Yan L Y, Jiang H F, Liu S Y, Lei Y, Liao B S. 2015b. Genome-wide analysis of the basic leucine zipper(bZIP)transcription factor gene family in six legume genomes. BMC Genomics, 16 (1):1053-1067.
doi: 10.1186/s12864-015-2258-x URL |
| [40] |
Wilkinson S W, Pastor V, Paplauskas S, P´etriacq P, Luna E. 2018. Long-lasting β-aminobutyric acid-induced resistance protects tomato fruit against Botrytis cinerea. Plant Pathology, 67 (1):30-41.
doi: 10.1111/ppa.2018.67.issue-1 URL |
| [41] | Withers J, Dong X N. 2016. Posttranslational modifications of NPR1:a single protein playing multiple roles in plant immunity and physiology. PLoS Pathogens, 12 (8):e1005707-e1005715. |
| [42] |
Zavaliev R, Mohan R, Chen T, Dong X. 2020. Formation of NPR1 condensates promotes cell survival during the plant immune response. Cell, 182 (5):1093-1108.
doi: S0092-8674(20)30881-3 pmid: 32810437 |
| [43] |
Zhang Y, Tessaro M J, Lassner M, Li X. 2003. Knockout analysis of Arabidopsis transcription factors TGA2,TGA5,and TGA6 reveals their redundant and essential roles in systemic acquired resistance. The Plant Cell, 15 (1):2647-2653.
doi: 10.1105/tpc.014894 URL |
| [44] |
Zhang Z K, Yang D P, Yang B, Gao Z Y, Li M, Jiang Y M, Hu M J. 2013. β-aminobutyric acid induces resistance of mango fruit to postharvest anthracnose caused by Colletotrichum gloeosporioides and enhances activity of fruit defense mechanisms. Scientia Horticulturae, 160 (1):78-84.
doi: 10.1016/j.scienta.2013.05.023 URL |
| [1] | 叶子茂, 申晚霞, 刘梦雨, 王 彤, 张晓楠, 余 歆, 刘小丰, 赵晓春, . R2R3-MYB转录因子CitMYB21对柑橘类黄酮生物合成的影响[J]. 园艺学报, 2023, 50(2): 250-264. |
| [2] | 宋艳红, 陈亚铎, 张晓玉, 宋 盼, 刘丽锋, 李 刚, 赵 霞, 周厚成, . 森林草莓FvbHLH130转录因子调控植株提前开花[J]. 园艺学报, 2023, 50(2): 295-306. |
| [3] | 韩 睿, 钟雄辉, 陈登辉, 崔 建, 乐祥庆, 颉建明, 康俊根, . 黑腐病菌效应因子XopR的甘蓝靶标基因BobHLH34的克隆及功能分析[J]. 园艺学报, 2023, 50(2): 319-330. |
| [4] | 田明康, 徐智祥, 刘秀群, 眭顺照, 李名扬, 李志能, . 蜡梅AP2亚家族转录因子鉴定及CpAP2-L11功能研究[J]. 园艺学报, 2023, 50(2): 382-396. |
| [5] | 翟含含, 翟宇杰, 田义, 张叶, 杨丽, 温陟良, 陈海江. 桃SAUR家族基因分析及PpSAUR5功能鉴定[J]. 园艺学报, 2023, 50(1): 1-14. |
| [6] | 袁馨, 徐云鹤, 张雨培, 单楠, 陈楚英, 万春鹏, 开文斌, 翟夏琬, 陈金印, 甘增宇. 猕猴桃后熟过程中ABA响应结合因子AcAREB1调控AcGH3.1的表达[J]. 园艺学报, 2023, 50(1): 53-64. |
| [7] | 蔺海娇, 梁雨晨, 李玲, 马军, 张璐, 兰振颖, 苑泽宁. 薰衣草CBF途径相关耐寒基因挖掘与调控网络分析[J]. 园艺学报, 2023, 50(1): 131-144. |
| [8] | 邢柱东, 吕福堂, 郭尚敬, 张演义. 新品种‘聊大红金’桃[J]. 园艺学报, 2023, 50(1): 225-226. |
| [9] | 杨兴旺, 王海波, 王莹莹, 王小龙, 王志强, 刘培培, 刘万春, 王孝娣. 中熟抗寒桃新品种‘中农甘爽’[J]. 园艺学报, 2022, 49(S2): 15-16. |
| [10] | 杨兴旺, 王海波, 王莹莹, 张艺灿, 王宝亮, 刘培培, 史祥宾, 刘万春, 王孝娣. 中熟抗寒桃新品种‘中农白干’[J]. 园艺学报, 2022, 49(S2): 17-18. |
| [11] | 杨兴旺, 刘凤之, 王海波, 王莹莹, 王志强, 史祥宾, 冀晓昊, 刘万春, 王孝娣. 中熟抗寒桃新品种‘中农寒水蜜’[J]. 园艺学报, 2022, 49(S2): 19-20. |
| [12] | 杨兴旺, 刘凤之, 王海波, 王莹莹, 张艺灿, 李 鹏, 王小龙, 刘万春, 王孝娣. 晚熟抗寒桃新品种‘中农秋香’[J]. 园艺学报, 2022, 49(S2): 21-22. |
| [13] | 王莹莹, 刘立常, 刘志伍, 杨兴旺, 刘万春, 王孝娣, . 极晚熟桃新品种‘中农冬蜜’[J]. 园艺学报, 2022, 49(S2): 23-24. |
| [14] | 王莹莹, 刘立常, 刘志伍, 杨兴旺, 刘万春, 王孝娣, . 小果油桃新品种‘中农珍珠’[J]. 园艺学报, 2022, 49(S2): 25-26. |
| [15] | 吴延军, 刘庆忠, 陈鸿才, 戚行江, 朱东姿, 郑家祥, 曹学敏, 方丹燕. 甜樱桃新品种‘江南锦’[J]. 园艺学报, 2022, 49(S2): 29-30. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司