
园艺学报 ›› 2021, Vol. 48 ›› Issue (12): 2385-2402.doi: 10.16420/j.issn.0513-353x.2020-1063
收稿日期:2021-04-19
修回日期:2021-07-21
发布日期:2022-01-04
通讯作者:
董春娟
E-mail:dongchunjuan@caas.cn
基金资助:
ZHANG Jingya, LEI Lei, SHANG Qingmao, XIE Lulu, DONG Chunjuan( )
)
Received:2021-04-19
Revised:2021-07-21
Published:2022-01-04
Contact:
DONG Chunjuan
E-mail:dongchunjuan@caas.cn
摘要:
从‘Ailsa Craig’番茄中克隆到两个IAA甲基转移酶(indole-3-acetic acid methyltransferase,IAMT)基因SlIAMT1和SlIAMT2,其全长分别为2 510和4 620 bp,均编码390个氨基酸。序列分析表明,二者均具有IAA甲基转移酶特异的底物结合位点和催化位点,与拟南芥AtIAMT1的序列相似度高于70%。利用PlantCARE数据库进行启动子顺式作用元件预测分析发现,SlIAMT1和SlIAMT2启动子序列中含有与生长素(IAA)、脱落酸(ABA)、水杨酸(SA)及光信号响应相关的顺式作用元件。荧光定量PCR分析表明,SlIAMT1在番茄萌发的种子、幼苗根、新叶以及青果中有优势表达,而SlIAMT2则主要在花蕾中表达。诱导表达分析表明,番茄幼苗中,尤其是下胚轴中,SlIAMT1的表达受强光照(150 μmol · m-2 · s-1)强烈诱导,而SlIAMT2受光照诱导程度较低;15 ℃低温处理对SlIAMT1和SlIAMT2的诱导程度均较低;IAA处理可显著诱导SlIAMT1和SlIAMT2的表达;ABA诱导根和下胚轴中SlIAMT1的表达,但抑制子叶中的SlIAMT1的表达,并抑制根中SlIAMT2的表达;SA抑制根和子叶中SlIAMT1的表达,但对子叶中SlIAMT2的表达有一定的诱导作用。异源转化结果显示,拟南芥iamt1缺失突变体下胚轴和主根长于野生型,突变体中过量表达SlIAMT1可有效恢复下胚轴和主根的表型;进一步分析表明,SlIAMT1过量表达显著降低了拟南芥幼苗侧根对IAA的敏感性。试验结果表明,SlIAMT响应光和激素信号,通过IAA甲基化修饰调节IAA稳态,调控幼苗下胚轴和根系发育。
中图分类号:
张静亚, 雷蕾, 尚庆茂, 谢露露, 董春娟. 番茄SlIAMT1和SlIAMT2的表达及其对下胚轴和根生长的调控[J]. 园艺学报, 2021, 48(12): 2385-2402.
ZHANG Jingya, LEI Lei, SHANG Qingmao, XIE Lulu, DONG Chunjuan. Expression Patterns of Tomato SlIAMT1 and SlIAMT2 and Their Functions During Hypocotyl and Root Development[J]. Acta Horticulturae Sinica, 2021, 48(12): 2385-2402.
| 用途Use | 基因Gene | 引物序列(5′-3′)Sequence |
|---|---|---|
| 基因克隆 Gene clone | SlIAMT1 | F:CACTAGCAAAACAATAGAGACA;R:TCTAATACATGAGAGACATATAGACTT |
| SlIAMT2 | F:TCTACTTTTATGGTTAGATTCTG;R:TATCCCAAATGTTGCTTAA | |
| qRT-PCR | SlIAMT1 | F:CTCAAGCTCAGGGGCAACAT;R:ATCTCCGGCGAGTTTAGCTG |
| SlIAMT2 | F:CTCAAGCCCAGGGACAACAT;R:CGAAGGGGATGTCGTCGTTAT | |
| SlActin41 | F:CTTCCAGCAGATGTGGATTGC;R:GCATCTCTGGTCCAGTAGGAAA | |
| AtIAMT1 | F:CGGTCTACTCTTCGGCACTC;R:GGTGCGTACACCGGGATATT | |
| AtActin2 | F:ACACTGTGCCAATCTACGAGGGTT;R:ACAATTTCCCGCTCTGCTGTTGTG | |
| 载体构建 Transgenic vector construction | 35S:SlIAMT1 | F:CGGTACCCGGGGATCCATGGCACCTTTAGGAGACAA; R:CGACTCTAGAGGATCCCTACACAAGAGAAAGTGAAGCAACA |
表1 本研究中所用的引物
Table 1 Primers used in this study
| 用途Use | 基因Gene | 引物序列(5′-3′)Sequence |
|---|---|---|
| 基因克隆 Gene clone | SlIAMT1 | F:CACTAGCAAAACAATAGAGACA;R:TCTAATACATGAGAGACATATAGACTT |
| SlIAMT2 | F:TCTACTTTTATGGTTAGATTCTG;R:TATCCCAAATGTTGCTTAA | |
| qRT-PCR | SlIAMT1 | F:CTCAAGCTCAGGGGCAACAT;R:ATCTCCGGCGAGTTTAGCTG |
| SlIAMT2 | F:CTCAAGCCCAGGGACAACAT;R:CGAAGGGGATGTCGTCGTTAT | |
| SlActin41 | F:CTTCCAGCAGATGTGGATTGC;R:GCATCTCTGGTCCAGTAGGAAA | |
| AtIAMT1 | F:CGGTCTACTCTTCGGCACTC;R:GGTGCGTACACCGGGATATT | |
| AtActin2 | F:ACACTGTGCCAATCTACGAGGGTT;R:ACAATTTCCCGCTCTGCTGTTGTG | |
| 载体构建 Transgenic vector construction | 35S:SlIAMT1 | F:CGGTACCCGGGGATCCATGGCACCTTTAGGAGACAA; R:CGACTCTAGAGGATCCCTACACAAGAGAAAGTGAAGCAACA |
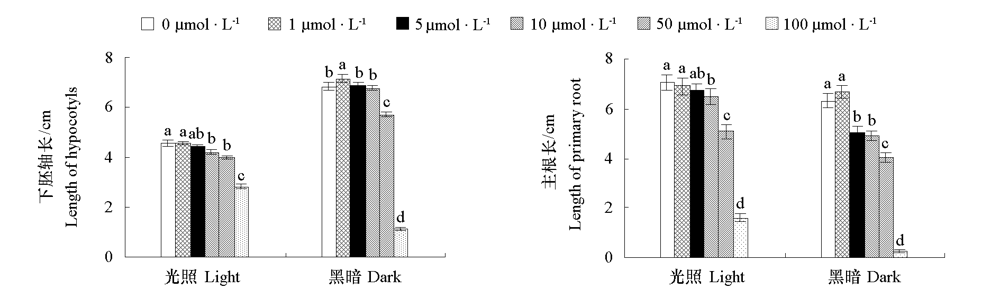
图1 光照和黑暗条件下外源MeIAA处理对番茄幼苗下胚轴和根系生长的影响 不同小写字母表示相同培养条件下差异显著(P < 0.05)。
Fig. 1 Effects of exogenous MeIAA application on growth of hypocotyls and roots in tomato seedlings under light and dark conditions Different lowercases indicate significant differences between groups under same culture condition(P < 0.05).
| 基因 Gene | 序列号 Accession No. | 基因全长/bp Gene length | 外显子数 Number of exons | 内含子数 Number of introns | ORF/ bp | 蛋白长度/aa Protein length | 分子量/ kD MW | 等电点 pI | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|---|
| SlIAMT1 | Solyc07G064990 | 2 510 | 4 | 3 | 1 173 | 390 | 42.92 | 5.58 | 细胞质Cytoplasm |
| SlIAMT2 | Solyc12G014500 | 4 620 | 4 | 3 | 1 173 | 390 | 43.28 | 5.90 | 细胞质Cytoplasm |
表2 SlIAMT1和SlIAMT2基因的序列特征
Table 2 Sequence characteristics of SlIAMT1 and SlIAMT2 genes in tomato
| 基因 Gene | 序列号 Accession No. | 基因全长/bp Gene length | 外显子数 Number of exons | 内含子数 Number of introns | ORF/ bp | 蛋白长度/aa Protein length | 分子量/ kD MW | 等电点 pI | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|---|
| SlIAMT1 | Solyc07G064990 | 2 510 | 4 | 3 | 1 173 | 390 | 42.92 | 5.58 | 细胞质Cytoplasm |
| SlIAMT2 | Solyc12G014500 | 4 620 | 4 | 3 | 1 173 | 390 | 43.28 | 5.90 | 细胞质Cytoplasm |
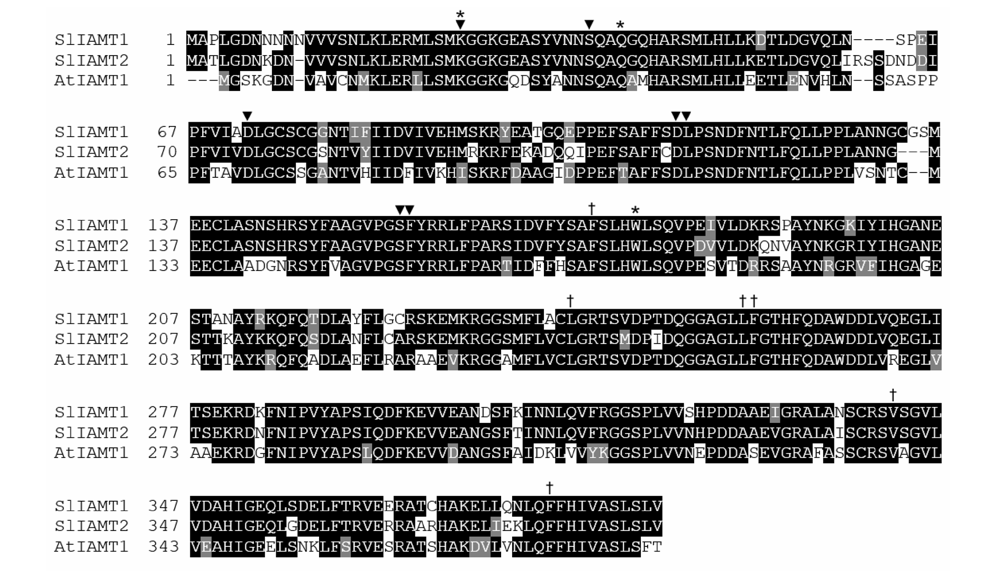
图2 SlIAMT1和SlIAMT2与拟南芥AtIAMT1蛋白序列的同源比对 ▼:SAM/SAH结合位点;*:与IAA羧基的作用位点;†:与IAA芳香基团的作用位点。
Fig. 2 Homology alignment of SlIAMT1 and SlIAMT2 with Arabidopsis AtIAMT1 protein sequences ▼:SAM/SAH-binding site;*:Interaction site with carboxyl moiety of IAA;†:Interaction site with aromatic moiety of IAA.
| 调控元件 Cis-element | 核心序列 Core sequence | 元件功能描述 Functional description of cis-element | 位置Positions* | |
|---|---|---|---|---|
| SlIAMT1 | SlIAMT2 | |||
| ABRE | CACGTG | ABA响应元件 cis-Acting element involved in the abscisic acid responsiveness | -1 547 | -552、 -1 129、 -1 921 |
| TCA-element | CCATCTTTTT | 水杨酸响应元件 cis-Acting element involved in salicylic acid responsiveness | -1 513 | / |
| TGA-element | AACGAC | 生长素响应元件 Auxin-responsive element | / | -791 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应元件 cis-Acting regulatory element involved in the methyl jasmonate responsiveness | / | -367 |
| MBS | CAACTG | 参与干旱诱导的MYB结合位点 MYB binding site involved in drought-inducibility | / | -712 |
| ACE | CTAACGTATT | 光响应元件cis-Acting element for light responsive | -1 754 | / |
| G-box | TACGTG | 光响应元件cis-Acting element for light responsive | -1 546,-1 981 | / |
| GT1-motif | GGTTAA | 光响应元件Light responsive element | -263,-170, -1 500,-1 810 | / |
| AE-box | AGAAACAA | 光响应元件的一部分 Part of a module for light response | -1 867 | / |
| AT1-motif | AATTATTTTTTATT | 光响应元件的一部分 Part of a light responsive module | -1 711、-698 | / |
| I-box | TGATAATGT | 光响应元件的一部分 Part of a light responsive element | -1 331 | / |
| MRE | AACCTAA | 参与光响应的MYB结合位点 MYB binding site for light responsive | / | -1 614、 -1 079 |
表3 SlIAMT1和SlIAMT2启动子重要顺式作用元件
Table 3 Some important cis-acting regulatory elements in the promoters of SlIAMT1 and SlIAMT2
| 调控元件 Cis-element | 核心序列 Core sequence | 元件功能描述 Functional description of cis-element | 位置Positions* | |
|---|---|---|---|---|
| SlIAMT1 | SlIAMT2 | |||
| ABRE | CACGTG | ABA响应元件 cis-Acting element involved in the abscisic acid responsiveness | -1 547 | -552、 -1 129、 -1 921 |
| TCA-element | CCATCTTTTT | 水杨酸响应元件 cis-Acting element involved in salicylic acid responsiveness | -1 513 | / |
| TGA-element | AACGAC | 生长素响应元件 Auxin-responsive element | / | -791 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应元件 cis-Acting regulatory element involved in the methyl jasmonate responsiveness | / | -367 |
| MBS | CAACTG | 参与干旱诱导的MYB结合位点 MYB binding site involved in drought-inducibility | / | -712 |
| ACE | CTAACGTATT | 光响应元件cis-Acting element for light responsive | -1 754 | / |
| G-box | TACGTG | 光响应元件cis-Acting element for light responsive | -1 546,-1 981 | / |
| GT1-motif | GGTTAA | 光响应元件Light responsive element | -263,-170, -1 500,-1 810 | / |
| AE-box | AGAAACAA | 光响应元件的一部分 Part of a module for light response | -1 867 | / |
| AT1-motif | AATTATTTTTTATT | 光响应元件的一部分 Part of a light responsive module | -1 711、-698 | / |
| I-box | TGATAATGT | 光响应元件的一部分 Part of a light responsive element | -1 331 | / |
| MRE | AACCTAA | 参与光响应的MYB结合位点 MYB binding site for light responsive | / | -1 614、 -1 079 |
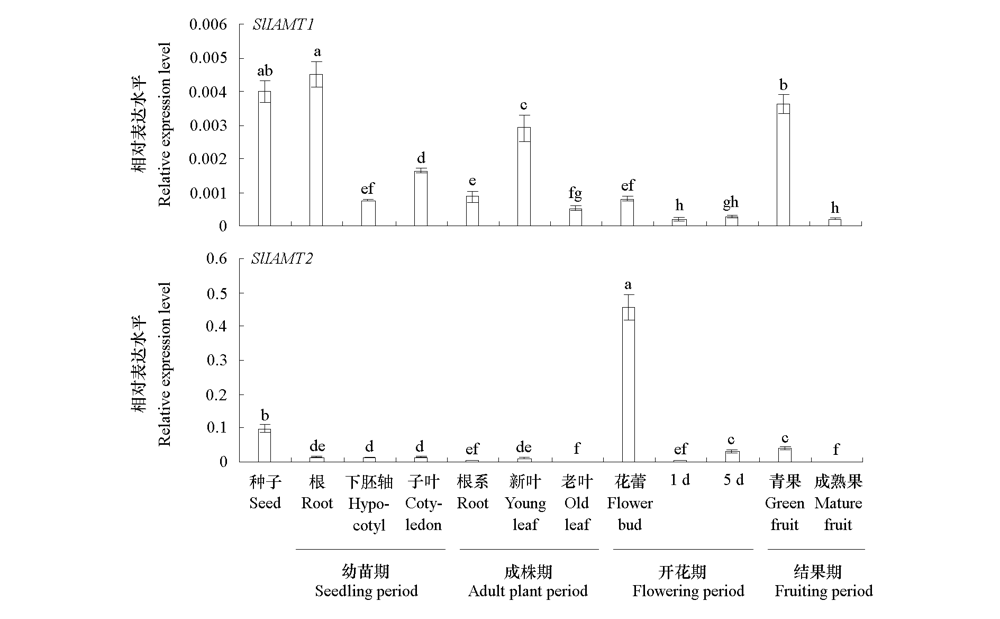
图4 番茄组织中SlIAMT1和SlIAMT2的表达 不同小写字母表示不同组织间差异显著(P < 0.05)。
Fig. 4 Expression levels of SlIAMT1 and SlIAMT2 in different tissues of tomato The different lowercases indicate significant differences between groups(P < 0.05).
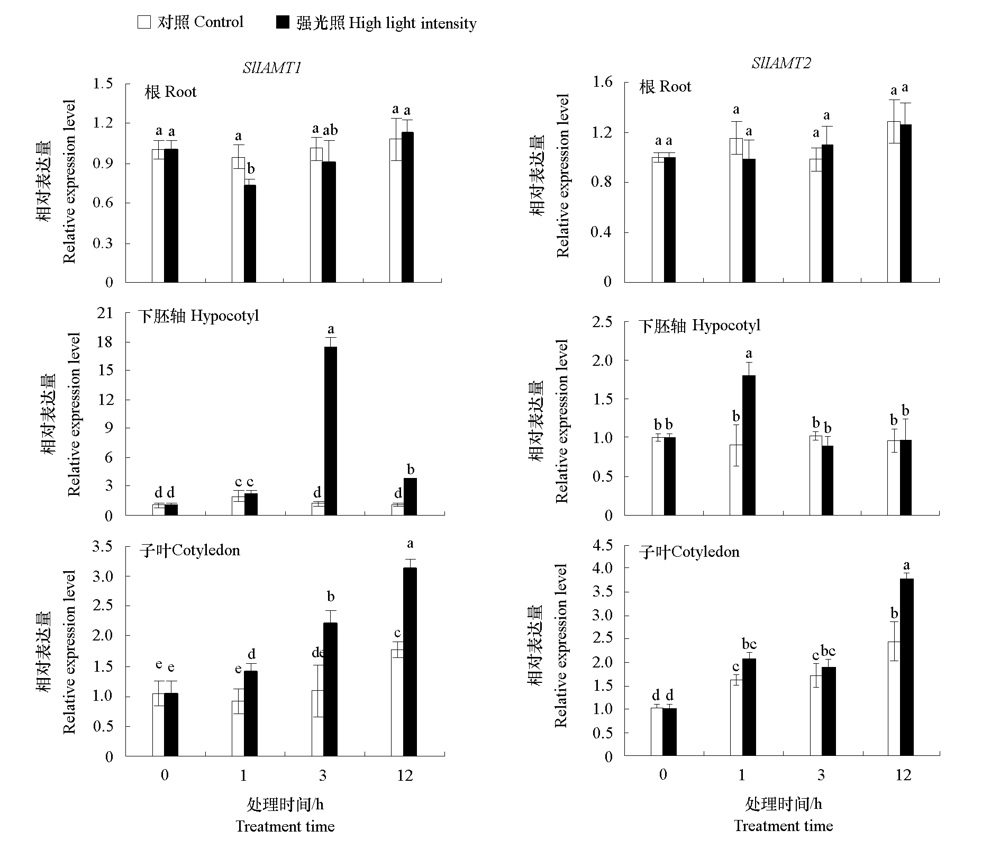
图5 番茄幼苗根、下胚轴和子叶中SlIAMT1 和SlIAMT2对强光照(150 μmol · m-2 · s-1)的响应 不同小写字母表示处理间差异显著(P < 0.05)。
Fig. 5 Expression levels of SlIAMT1 and SlIAMT2 in root,hypocotyl,and cotyledon of tomato seedlings in response to high light intensity(150 μmol · m-2 · s -1 Different lowercases indicate significant differences between groups(P < 0.05).
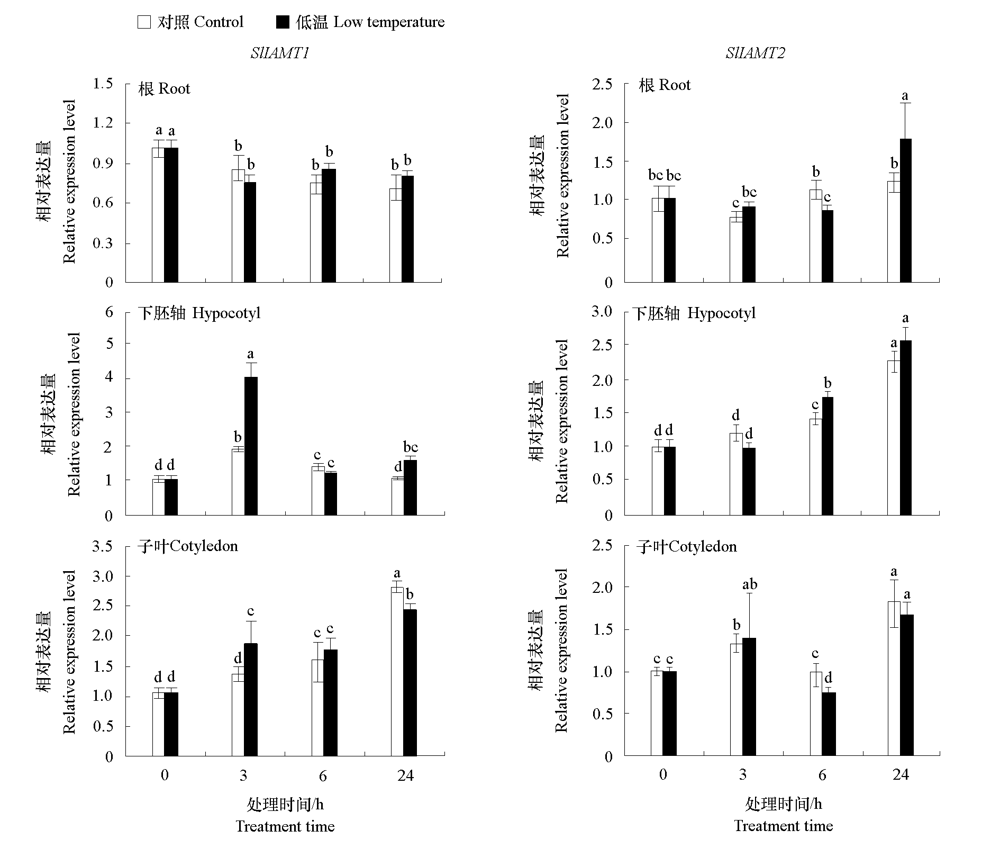
图6 番茄幼苗根、下胚轴和子叶中SlIAMT1和SlIAMT2对低温(15 ℃/15 ℃)的响应 不同小写字母表示处理间差异显著(P < 0.05)。
Fig. 6 Expression levels of SlIAMT1 and SlIAMT2 in root,hypocotyl,and cotyledon of tomato seedlings in response to low temperature(15 ℃/15 ℃) Different lowercases indicate significant differences between groups(P < 0.05).
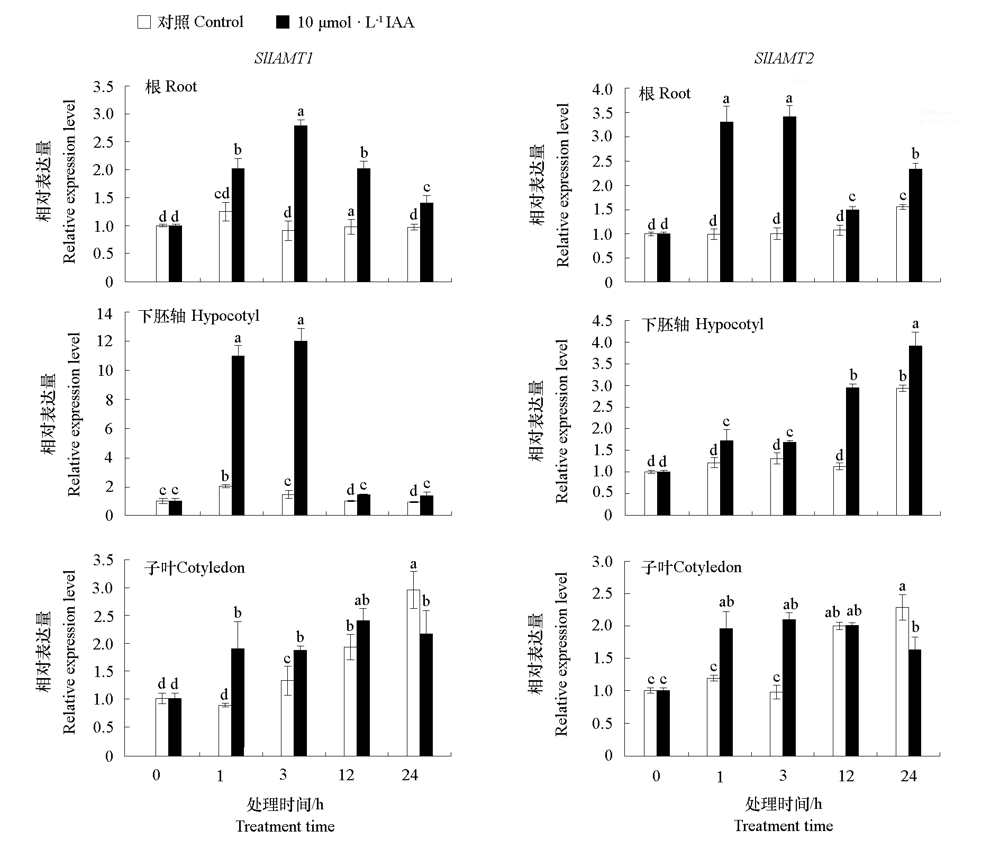
图7 番茄幼苗根、下胚轴和子叶中SlIAMT1 和SlIAMT2对IAA的响应 不同小写字母表示处理间差异显著(P < 0.05)。
Fig. 7 Expression levels of SlIAMT1 and SlIAMT2 in root,hypocotyl,and cotyledonof tomato seedlings in response to IAA Different lowercases indicate significant differences between groups(P < 0.05).
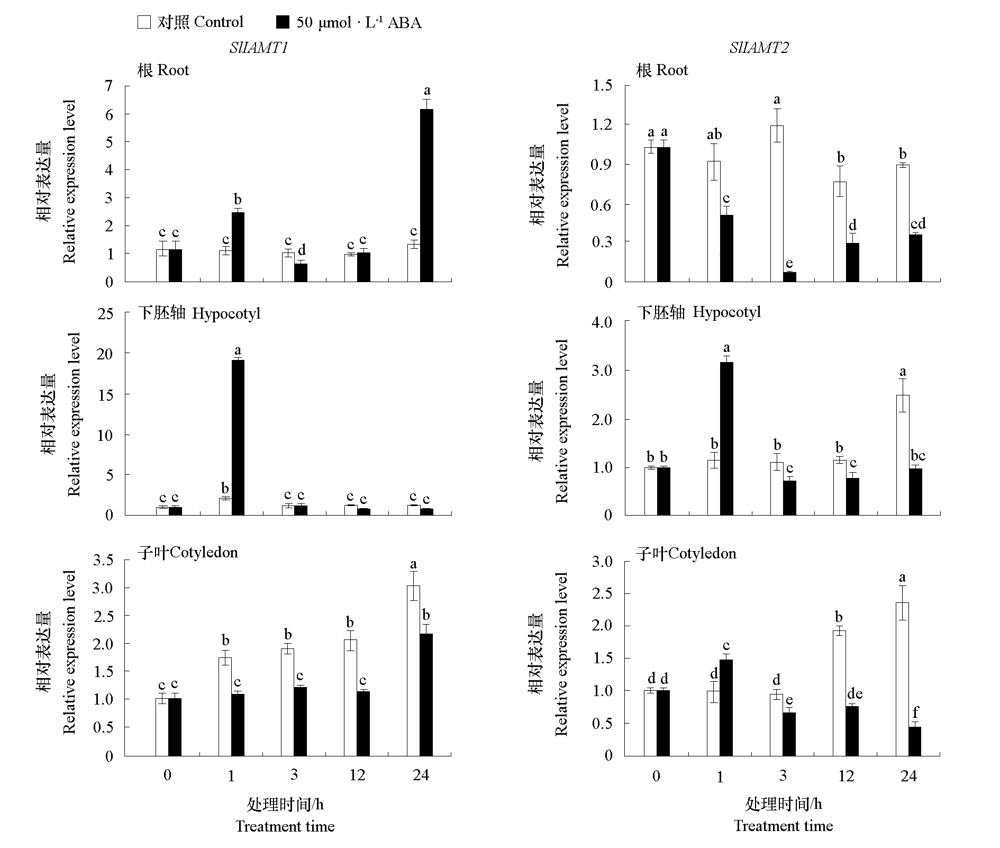
图8 番茄幼苗根、下胚轴和子叶中SlIAMT1 和SlIAMT2对ABA的响应 不同小写字母表示处理间差异显著(P < 0.05)。
Fig. 8 Expression levels of SlIAMT1 and SlIAMT2 in root,hypocotyl,and cotyledon of tomato seedlings in response to ABA Different lowercases indicate significant differences between groups(P < 0.05).
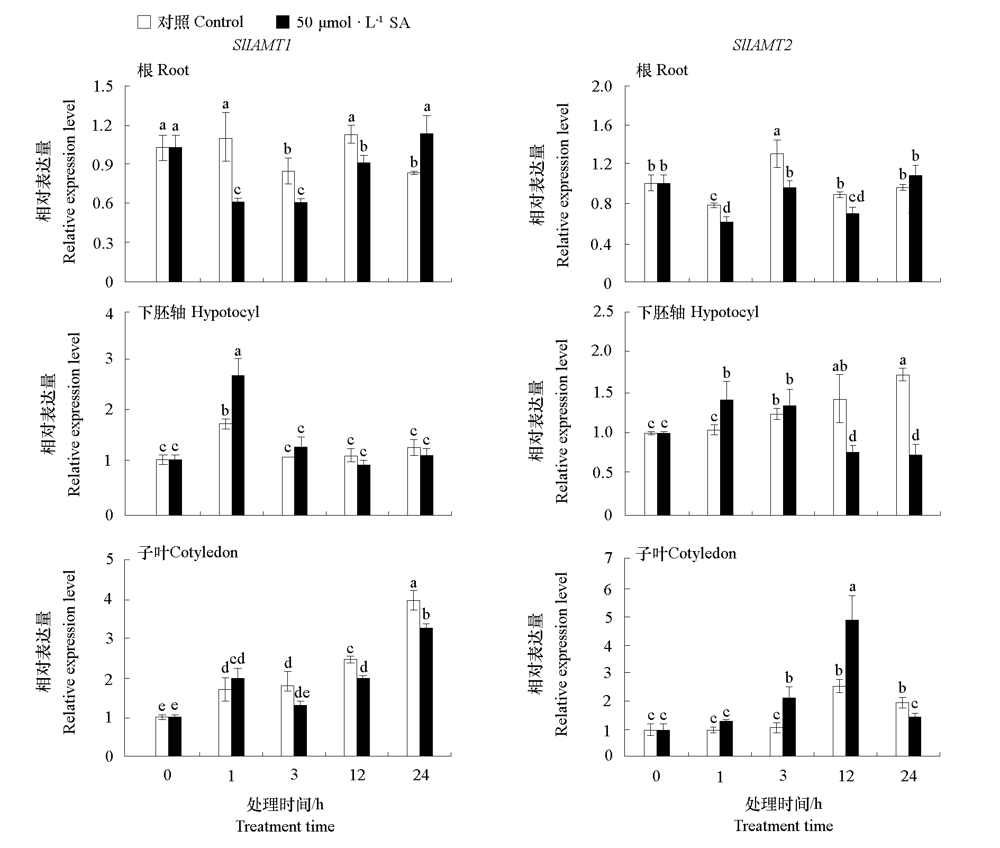
图9 番茄幼苗根、下胚轴和子叶中SlIAMT1和SlIAMT2对SA的响应 不同小写字母表示处理间差异显著(P < 0.05)。
Fig. 9 Expression levels of SlIAMT1 and SlIAMT2 in root,hypocotyl,and cotyledon of tomato seedlings in response to SA Different lowercases indicate significant differences between groups(P < 0.05).
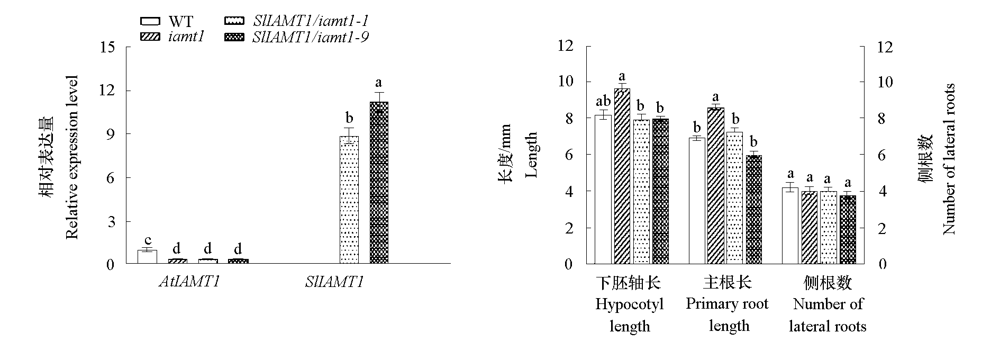
图10 SlIAMT1过量表达对拟南芥iamt1突变体幼苗下胚轴和根系生长的影响 不同小写字母表示差异显著(P < 0.05)。
Fig. 10 Effects of SlIAMT1 overexpression on hypocotyl and root growth in Arabidopsis iamt1 mutant Different lowercases indicate significant differences between groups(P < 0.05).
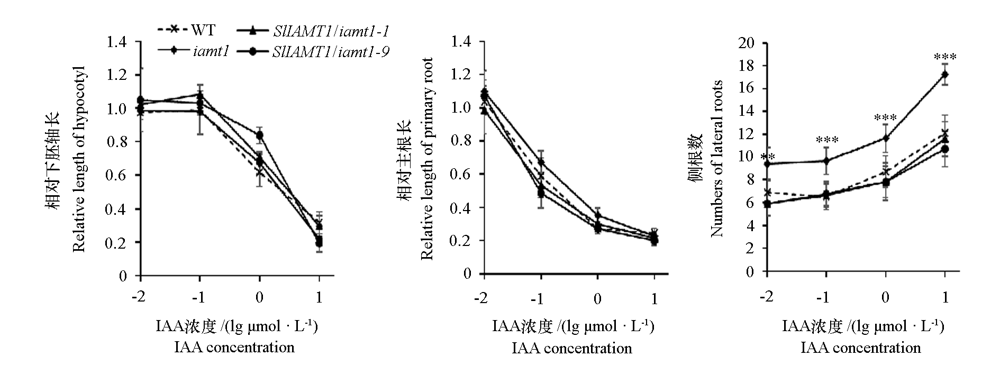
图11 SlIAMT1过量表达对拟南芥iamt1突变体幼苗响应IAA的影响 IAA浓度是取lg后的值。**、***分别表示在0.01和0.001水平差异显著。
Fig. 11 Effects of overexpression of SlIAMT1 on the response of Arabidopsis iamt1 mutant seedlings to IAA The concentration of IAA is the logarithmic value. **,*** indicate significant differences between groups at 0.01 and 0.001 level,respectively.
| [1] |
Abbas M, Hernández-García J, Blanco-Touriñán N, Aliaga N, Minguet E G, Alabadí D, Blázquez M A. 2018a. Reduction of indole-3-acetic acid methyltransferase activity compensates for high-temperature male sterility in Arabidopsis. Plant Biotechnology Journal, 16 (1):272-279.
doi: 10.1111/pbi.2018.16.issue-1 URL |
| [2] | Abbas M, Hernández-García J, Pollmann S, Samodelov S L, Kolb M, Friml J, Hammes U Z, Zurbriggen M D, Blázquez M A, Alabadí D. 2018b. Auxin methylation is required for differential growth in Arabidopsis. Proceedings of the National Academy of Sciences, 115 (26):6864-6869. |
| [3] |
Aloni R. 2013. Role of hormones in controlling vascular differentiation and the mechanism of lateral root initiation. Planta, 238 (5):819-830.
doi: 10.1007/s00425-013-1927-8 pmid: 23835810 |
| [4] |
An J P, Liu X, Song L Q, You C X, Hao Y J. 2017. Functional characterization of the apple RING E 3 ligase MdMIEL1 in transgenic Arabidopsis. Horticultural Plant Journal, 3 (2):53-59.
doi: 10.1016/j.hpj.2017.01.001 URL |
| [5] |
Chhun T, Taketa S, Tsurumi S, Ichii M. 2003. The effects of auxin on lateral root initiation and root gravitropism in a lateral rootless mutant Lrt1 of rice(Oryza sativa L.). Plant Growth Regulation, 39:161-170.
doi: 10.1023/A:1022592511387 URL |
| [6] |
Chung M H, Chen M K, Pan S M. 2000. Floral spray transformation can efficiently generate Arabidopsis transgenic plants. Transgenic Research, 9 (6):471-476.
pmid: 11206976 |
| [7] |
Collett C E, Harberd N P, Leyser O. 2000. Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiology, 124 (2):553-561.
pmid: 11027706 |
| [8] | Dias M C, Oliveira H, Costa A, Santos C. 2014. Improving elms performance under drought stress:the pretreatment with abscisic acid. Environmental & Experimental Botany, 100:64-73. |
| [9] | Ding Mao-yu, Hou Xian-hui, Liu Sai-nan, Li Lin-chuan, Chen Zhang-liang, Kang Ding-ming, Qu Li-jia. 2013. Study on expression pattern of Arabidopsis MeIAA esterase genes. Journal of China Agricultural University, 18 (2):1-8. (in Chinese) |
| 丁茂予, 侯仙慧, 刘赛男, 李林川, 陈章良, 康定明, 瞿礼嘉. 2013. 拟南芥MeIAA酯解酶基因表达模式的初步研究. 中国农业大学学报, 18 (2):1-8. | |
| [10] |
Du M, Spalding E P, Gray W M. 2020. Rapid auxin-mediated cell expansion. Annual Review of Plant Biology, 71 (1):1-24.
doi: 10.1146/annurev-arplant-081519-035831 URL |
| [11] |
Feng Han-qian, Li Chao. 2018. Research advances of auxin signal transduction. Biotechnology Bulletin, 34 (7):24-30. (in Chinese)
doi: 10.13560/j.cnki.biotech.bull.1985.2018-0488 |
|
冯寒骞, 李超. 2018. 生长素信号转导研究进展. 生物技术通报, 34 (7):24-30.
doi: 10.13560/j.cnki.biotech.bull.1985.2018-0488 |
|
| [12] |
Gallei M, Luschnig C, Friml J. 2020. Auxin signalling in growth:Schrödinger's cat out of the bag. Current Opinion in Plant Biology, 53:43-49.
doi: 10.1016/j.pbi.2019.10.003 URL |
| [13] |
Han W X, Han D L, He Z P, Hu H, Wu Q, Zhang J J, Jiang J M, Qin G J, Cui Y H, Lai J B, Yang C W. 2018. The SWI/SNF subunit SWI3B regulates IAMT1 expression via chromatin remodeling in Arabidopsis leaf development. Plant Science, 271:127-132.
doi: 10.1016/j.plantsci.2018.03.021 URL |
| [14] | Hou Xian-hui, Ding Mao-yu, Liu Sai-nan, Li Lin-chuan, Qu Li-jia. 2009. Isolation and positional cloning of methyl-indole-3-aceticacid resistant mutants in Arabidopsis. Chinese Bulletin of Botany, 44 (1):52-58. (in Chinese) |
| 侯仙慧, 丁茂予, 刘赛男, 李林川, 瞿礼嘉. 2009. 拟南芥MeIAA抗性突变体的筛选和初步图位克隆分析. 植物学报, 44 (1):52-58. | |
| [15] |
Kolosova N, Sherman D, Karlson D, Dudareva N. 2001. Cellular and subcellular localization of S-adenosyl-L-methionine:benzoic acid carboxyl methyltransferase,the enzyme responsible for biosynthesis of the volatile ester methylbenzoate in snapdragon flowers. Plant Physiology, 126:956-964.
pmid: 11457946 |
| [16] |
Lam K C, Ibrahim R K, Behdad B, Dayanandan S. 2007. Structure,function,and evolution of plant O-methyltransferases. Genome, 50:1001-1013.
doi: 10.1139/G07-077 URL |
| [17] |
Li L, Hou X H, Tsuge T, Ding M Y, Aoyama T, Oka A, Gu H Y, Zhao Y D, Qu L J. 2008. The possible action mechanisms of indole-3-acetic acid methyl ester in Arabidopsis. Plant Cell Reports, 27 (3):575-584.
doi: 10.1007/s00299-007-0458-9 URL |
| [18] | Li Lin-chuan, Qu Li-jia. 2006. Regulation of leaf development by auxin in Arabidopsis. Chinese Bulletin of Botany, 23 (5):459-465. (in Chinese) |
| 李林川, 瞿礼嘉. 2006. 生长素对拟南芥叶片发育调控的研究进展. 植物学通报, 23 (5):459-465. | |
| [19] | Li Min, Yang Shuang, Ruan Yan-ye, Fan Jin-juan, Zhang Li-jun. 2006. Identification of ATSUC3 with T-DNA insertion by PCR. Plant Physiology Communications, 42 (1):91-94. (in Chinese) |
| 李敏, 杨双, 阮燕晔, 樊金娟, 张立军. 2006. 拟南芥T-DNA插入突变体ATSUC3的PCR鉴定. 植物生理学通讯, 42 (1):91-94. | |
| [20] | Li Shuang. 2012. Preliminary study of regulation of phytohormone methylation in Arabidopsis growth and development[M. D. Dissertation]. Beijing: Peking University. (in Chinese) |
| 李爽. 2012. 植物激素甲基化修饰调控拟南芥生长发育的初步研究[硕士论文]. 北京: 北京大学. | |
| [21] | Li Shu-yu, Li Chuan-you. 2016. Developmental plasticity of plant roots. China Basic Science, 18 (2):14-21. (in Chinese) |
| 李淑钰, 李传友. 2016. 植物根系可塑性发育的研究进展与展望. 中国基础科学, 18 (2):14-21. | |
| [22] | Liu J, Shi M, Wang J, Zhang B, Li Y, Wang J, El-Sappah A H, Liang Y. 2020. Comparative transcriptomic analysis of the development of sepal morphology in tomato(Solanum lycopersicum L.). International Journal of Molecular Sciences, 21 (16):E5914. |
| [23] |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25 (4):402-408.
pmid: 11846609 |
| [24] |
Ljung K. 2013. Auxin metabolism and homeostasis during plant development. Development, 140 (5):943-950.
doi: 10.1242/dev.086363 URL |
| [25] |
Park J E, Park J Y, Kim Y S, Staswick P E, Jeon J, Yun J, Kim S Y, Kim J, Lee Y H, Park C M. 2007. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. Journal of Biological Chemistry, 282 (13):10036-10046.
doi: 10.1074/jbc.M610524200 URL |
| [26] |
Qin G J, Gu H Y, Zhao Y D, Ma Z Q, Shi G L, Yang Y, Pichersky E, Chen H D, Liu M H, Chen Z L, Qu L J. 2005. An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell, 17 (10):2693-2704.
doi: 10.1105/tpc.105.034959 URL |
| [27] |
Qu L J, Li S, Xing S F. 2010. Methylation of phytohormones by the SABATH methyltransferases. Chinese Science Bulletin, 55 (21):2211-2218.
doi: 10.1007/s11434-010-3245-x URL |
| [28] | Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor E B. 2007. Auxin,actin and growth of the Arabidopsis thaliana primary root. Plant Journal for Cell & Molecular Biology, 50 (3):514. |
| [29] | Roychoudhury A, Paul S, Basu S. 2013. Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell, 32 (7):985-1006. |
| [30] |
Takubo E, Kobayashi M, Hirai S, Aoi Y, Ge C, Dai X, Fukui K, Hayashi K I, Zhao Y, Kasahara H. 2020. Role of Arabidopsis indole-3-acetic acid carboxyl methyltransferase 1 in auxin metabolism. Biochemical and Biophysical Research Communications, 527 (4):1033-1038.
doi: S0006-291X(20)30938-4 pmid: 32444138 |
| [31] |
Vanneste S, Friml J. 2009. Auxin:a trigger for change in plant development. Cell, 136 (6):1005-1016.
doi: 10.1016/j.cell.2009.03.001 pmid: 19303845 |
| [32] |
Vilches-Barro A, Maizel A. 2015. Talking through walls:mechanisms of lateral root emergence in Arabidopsis thaliana. Current Opinion in Plant Biology, 23:31-38.
doi: 10.1016/j.pbi.2014.10.005 pmid: 25449724 |
| [33] |
Weijers D, Wagner D. 2016. Transcriptional responses to the auxin hormone. Annual Review of Plant Biology, 67 (1):539-574.
doi: 10.1146/arplant.2016.67.issue-1 URL |
| [34] |
Woodward A W, Bartel B. 2005. Auxin:regulation,action,and interaction. Annals of Botany, 95 (5):707-735.
pmid: 15749753 |
| [35] |
Yang Y, Xu R, Ma C J, Vlot A C, Klessig D F, Pichersky E. 2008. Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis. Plant Physiology, 147 (3):1034-1045.
doi: 10.1104/pp.108.118224 pmid: 18467465 |
| [36] |
Zhang J, Peer W A. 2017. Auxin homeostasis:the DAO of catabolism. Journal of Experimental Botany, 68 (12):3145-3154.
doi: 10.1093/jxb/erx221 pmid: 28666349 |
| [37] |
Zhang Y L, Li X. 2019. Salicylic acid:biosynthesis,perception,and contributions to plant immunity. Current Opinion in Plant Biology, 50:29-36.
doi: 10.1016/j.pbi.2019.02.004 URL |
| [38] |
Zhang Z L, Ji R H, Li H Y, Zhao T, Liu J, Lin C T, Liu B. 2014. CONSTANS-LIKE 7 (COL7)is involved in phytochrome B (phyB)-mediated light-quality regulation of auxin homeostasis. Molecular Plant, 7 (9):1429-1440.
doi: 10.1093/mp/ssu058 URL |
| [39] |
Zhao N, Ferrer J L, Ross J, Guan J, Yang Y, Pichersky E, Noel J P, Chen F. 2008. Structural,biochemical,and phylogenetic analyses suggest that indole-3-acetic acid methyltransferase is an evolutionarily ancient member of the SABATH family. Plant Physiology, 146 (2):455-467.
pmid: 18162595 |
| [40] |
Zhao N, Guan J, Lin H, Chen F. 2007. Molecular cloning and biochemical characterization of indole-3-acetic acid methyltransferase from poplar. Phytochemistry, 68 (11):1537-1544.
pmid: 17499822 |
| [41] |
Zhao Y D. 2010. Auxin biosynthesis and its role in plant development. Annual Review of Plant Biology, 61:49-64.
doi: 10.1146/arplant.2010.61.issue-1 URL |
| [42] | Zou Li-ping, Pan Cheng, Wang Meng-xin, Cui Lin, Han Bao-yu. 2020. Progress on the mechanism of hormones regulating plant flower formation. Hereditas(Beijing), 42 (8):739-751. |
| 邹礼平, 潘铖, 王梦馨, 崔林, 韩宝瑜. 2020. 激素调控植物成花机理研究进展. 遗传, 42 (8):739-751. | |
| [43] |
Zubieta C, Ross J R, Koscheski P, Yang Y, Pichersky E, Noel J P. 2003. Structural basis for substrate recognition in the salicylic acid carboxyl methyltransferase family. Plant Cell, 15:1704-1716.
pmid: 12897246 |
| [1] | 史洪丽, 李腊, 郭翠梅, 余婷婷, 简伟, 杨星勇. 番茄灰霉病生防菌株TL1的分离、鉴定及其生防能力分析[J]. 园艺学报, 2023, 50(1): 79-90. |
| [2] | 忽靖宇, 阙开娟, 缪田丽, 吴少政, 王田田, 张磊, 董鲜, 季鹏章, 董家红. 侵染鸢尾的番茄斑萎病毒鉴定[J]. 园艺学报, 2023, 50(1): 170-176. |
| [3] | 赵雪艳, 王琪, 王莉, 王方圆, 王庆, 李艳. 基于比较转录组的延胡索组织差异性表达分析[J]. 园艺学报, 2023, 50(1): 177-187. |
| [4] | 郑积荣, 王同林, 胡松申. 高品质番茄新品种‘杭杂603’[J]. 园艺学报, 2022, 49(S2): 103-104. |
| [5] | 郑积荣, 王同林. 番茄新品种‘杭杂601’[J]. 园艺学报, 2022, 49(S2): 105-106. |
| [6] | 郑积荣, 王同林. 樱桃番茄新品种‘杭杂503’[J]. 园艺学报, 2022, 49(S2): 107-108. |
| [7] | 黄婷婷, 刘淑芹, 张永志, 李 平, 张志焕, 宋立波. 樱桃番茄新品种‘樱莎红4号’[J]. 园艺学报, 2022, 49(S2): 109-110. |
| [8] | 张前荣, 李大忠, 裘波音, 林 珲, 马慧斐, 叶新如, 刘建汀, 朱海生, 温庆放. 设施番茄新品种‘闽农科2号’[J]. 园艺学报, 2022, 49(S1): 73-74. |
| [9] | 韩帅, 吴婕, 张河庆, 席亚东. 四川莴笋上番茄斑萎病毒的电镜观察与小RNA测序鉴定[J]. 园艺学报, 2022, 49(9): 2007-2016. |
| [10] | 高彦龙, 吴玉霞, 张仲兴, 王双成, 张瑞, 张德, 王延秀. 苹果ELO家族基因鉴定及其在低温胁迫下的表达分析[J]. 园艺学报, 2022, 49(8): 1621-1636. |
| [11] | 邱子文, 刘林敏, 林永盛, 林晓洁, 李永裕, 吴少华, 杨超. 千层金MbEGS基因的克隆与功能分析[J]. 园艺学报, 2022, 49(8): 1747-1760. |
| [12] | 陈礼浪, 杨天章, 蔡儒平, 林小漫, 邓南康, 车海彦, 林雅婷, 孔祥义. 海南西番莲主要病毒种类的分子检测与鉴定[J]. 园艺学报, 2022, 49(8): 1785-1794. |
| [13] | 郑林, 王帅, 刘语诺, 杜美霞, 彭爱红, 何永睿, 陈善春, 邹修平. 柑橘响应黄龙病菌侵染的NAC基因的克隆及表达分析[J]. 园艺学报, 2022, 49(7): 1441-1457. |
| [14] | 马维峰, 李艳梅, 马宗桓, 陈佰鸿, 毛娟. 苹果POD家族基因的鉴定与MdPOD15的功能分析[J]. 园艺学报, 2022, 49(6): 1181-1199. |
| [15] | 张凯, 麻明英, 王萍, 李益, 金燕, 盛玲, 邓子牛, 马先锋. 柑橘HSP20家族基因鉴定及其响应溃疡病菌侵染表达分析[J]. 园艺学报, 2022, 49(6): 1213-1232. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司