
园艺学报 ›› 2021, Vol. 48 ›› Issue (11): 2197-2210.doi: 10.16420/j.issn.0513-353x.2020-1078
任文静, 于海龙, 陈立, 张斌, 陈文迪, 方智远, 杨丽梅, 庄木, 吕红豪, 王勇, 季家磊, 张扬勇( )
)
收稿日期:2021-02-25
修回日期:2021-08-20
发布日期:2021-12-02
基金资助:
REN Wenjing, YU Hailong, CHEN Li, ZHANG Bin, CHEN Wendi, FANG Zhiyuan, YANG Limei, ZHUANG Mu, LÜ Honghao, WANG Yong, JI Jialei, ZHANG Yangyong( )
)
Received:2021-02-25
Revised:2021-08-20
Published:2021-12-02
摘要:
为创制甘蓝Ogura细胞质雄性不育(CMS)育性恢复材料,在芥蓝和甘蓝型油菜远缘杂交后,采用甘蓝进行回交,构建BC4和BC5群体;基于Ogura CMS恢复基因Rfo编码区及其启动子序列,设计开发了适用于高分辨率熔解曲线(HRM)分析的特异InDel分子标记Rfo-11F/Rfo-11R;利用该标记对BC4和BC5代群体进行Rfo基因分型。从8 036株BC4回交后代中筛选获得Rfo基因阳性后代41株,平均Rfo基因传递率为0.51%,平均花粉活力为81%。从BC4代挑选5个重点Rfo阳性单株,细胞学观察结果显示5个单株的花粉母细胞染色体在减数分裂时期正常配对和分离的平均比例分别为84%和69%;进一步回交后,从15 408株BC5回交后代中获得Rfo阳性后代117株,Rfo基因传递率为0.75%。平均花粉活力为83%,7个重点BC5代Rfo阳性单株的花粉母细胞染色体在减数分裂时期配对和分离正常的平均比例为81%和84%。经过5代回交,甘蓝Rfo育性恢复系的倍性全部回复为正常的二倍体。所有Rfo阳性单株开花后育性表现为可育,与Rfo基因的分子标记鉴定结果完全一致,由此表明,基于本研究中开发标记构建的快速、准确、高效的Rfo基因高通量分型体系,可用于进一步回交转育后代筛选,大大提高育种效率。
中图分类号:
任文静, 于海龙, 陈立, 张斌, 陈文迪, 方智远, 杨丽梅, 庄木, 吕红豪, 王勇, 季家磊, 张扬勇. 甘蓝Ogura CMS育性恢复基因Rfo的传递效率解析及育种应用[J]. 园艺学报, 2021, 48(11): 2197-2210.
REN Wenjing, YU Hailong, CHEN Li, ZHANG Bin, CHEN Wendi, FANG Zhiyuan, YANG Limei, ZHUANG Mu, LÜ Honghao, WANG Yong, JI Jialei, ZHANG Yangyong. High-throughput Screening of Transmission Rate for Ogura CMS Fertility Restorer Gene Rfo by HRM Technique in Cabbage[J]. Acta Horticulturae Sinica, 2021, 48(11): 2197-2210.
| 引物名称 Primer name | 引物序列(5′-3′) Primer sequence | 片段大小/bp Fragment size | 参考文献 Reference |
|---|---|---|---|
| BnRFO-AS2F/BnRFO-NEW-R | F:CATGCTTCGATCTCGTCCTTTA; R:TACGACATTGGGCCTACATGTC | 500 | Yu et al., |
| Rfo-6eF/Rfo-6eR | F:AGCGGAGAGAGTTGCGAAGCAGGTT; R:CCATAAGTAATCTGGGTAGGCTGGAG | 566 | |
| Rfo-8F/Rfo-8R | F:CGGAACTCTACTGGTTTCAATAGCTCGG; R:GCCGCAGACTCAGCAGGAGAAGAAGAAC | 320 | |
| Rfo-11F/Rfo-11R | F:TATAAGCACGGCTCTACCTATGA; R:GAAACACTCACGAAATGCTACA | 300 | |
| Rfo-page-4eF/Rfo-page-4eR | F:TGAGTCTGCGGCTAGATTGTTCTGT; R:TTTCATGAAACCCACTTTGCAGCTT | 125 |
表1 本试验中使用的InDel引物
Table 1 Primers used for PCR in this study
| 引物名称 Primer name | 引物序列(5′-3′) Primer sequence | 片段大小/bp Fragment size | 参考文献 Reference |
|---|---|---|---|
| BnRFO-AS2F/BnRFO-NEW-R | F:CATGCTTCGATCTCGTCCTTTA; R:TACGACATTGGGCCTACATGTC | 500 | Yu et al., |
| Rfo-6eF/Rfo-6eR | F:AGCGGAGAGAGTTGCGAAGCAGGTT; R:CCATAAGTAATCTGGGTAGGCTGGAG | 566 | |
| Rfo-8F/Rfo-8R | F:CGGAACTCTACTGGTTTCAATAGCTCGG; R:GCCGCAGACTCAGCAGGAGAAGAAGAAC | 320 | |
| Rfo-11F/Rfo-11R | F:TATAAGCACGGCTCTACCTATGA; R:GAAACACTCACGAAATGCTACA | 300 | |
| Rfo-page-4eF/Rfo-page-4eR | F:TGAGTCTGCGGCTAGATTGTTCTGT; R:TTTCATGAAACCCACTTTGCAGCTT | 125 |
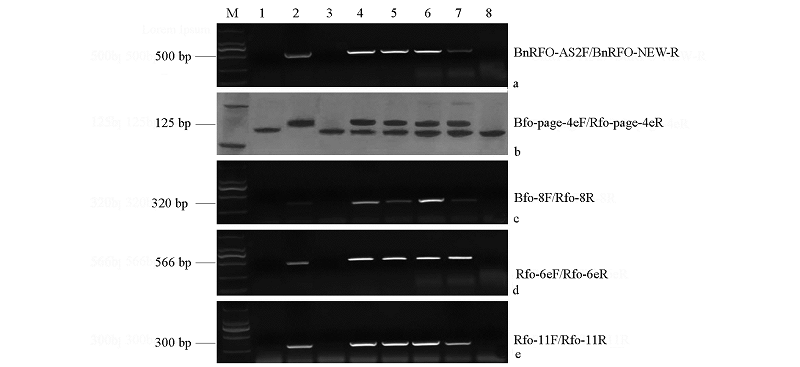
图2 5对引物的扩增产物在琼脂糖或聚丙烯酰胺凝胶上的电泳结果 1:母本芥蓝Y101;2:父本甘蓝型油菜Y403;3:BC2后代16Q2-4;4:F1后代YL2-1;5:BC1后代15CMSF-Y1;6:BC2后代16Q2-11;7:BC3后代F8-839;8:BC3后代F8-367;2、4、5、6、7为Rfo阳性单株。
Fig. 2 Amplification results of five pairs of primers in this study 1:Female parent Y101;2:Male parent Y403;3:16Q2-4 in BC2 generation;4:YL2-1 in F1 generation;5:15CMSF-Y1 in BC1 generation;6:16Q2-11 in BC2 generation;7:F8-839 in BC3 generation;8:F8-367 in BC3 generation;2,4,5,6,7 are Rfo-positive cabbages.
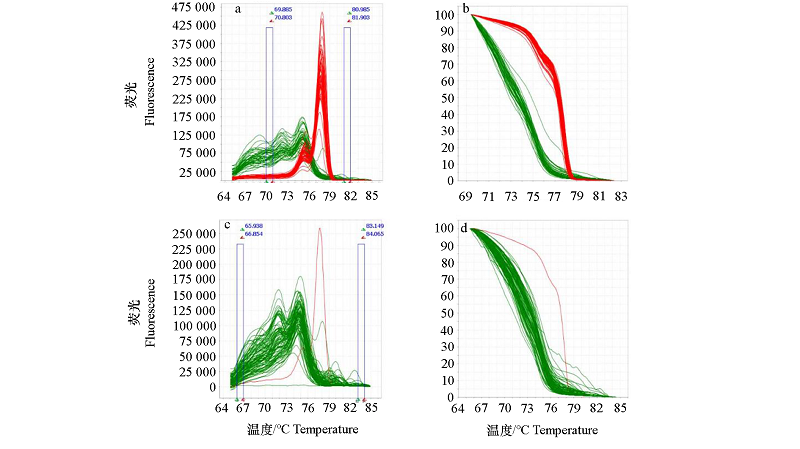
图3 引物Rfo-11F/Rfo-11R的高通量分型结果 a和b:49株阳性株和47株阴性株的检测熔解曲线峰值转换结果图和检测熔解曲线图;c和d:自交系和雄性不育系的检测熔解曲线峰值转换结果图和熔解曲线图。
Fig. 3 Genotyping results of primer Rfo-11F/Rfo-11R by a high-throughput genotyping platform a and b:Peak conversion diagram of melt curve and melting curve diagram of 49 Rfo-positive and 47 Rfo-negative cabbages;c and d:Peak conversion diagram of melt curve and melting curve diagram of inbred lines and male sterile lines.
| 新编号 New code | 组合 Combination | 授粉花蕾数 Number of pollinated flowers | 角果数 Number of siliques | 种子数 Number of seeds | 结实率 Seed setting rate | 成苗数 Number of viable plants | Rfo阳性株 Number of Rfo-positive plants | Rfo传递率/% Transmission rate of Rfo in backcross progenies |
|---|---|---|---|---|---|---|---|---|
| 17GL1 × F8-839 | 19 | 10 | 137 | 13.7 | 0 | |||
| 18QR13 | 17GL2 × F9-1118 | 184 | 171 | 2 736 | 16.0 | 470 | 1 | 0.21 |
| 18QR14 | 17GL2 × F8-514 | 39 | 22 | 284 | 12.9 | 0 | ||
| 18QR15 | 17GL3 × F8-514 | 26 | 13 | 239 | 18.4 | 188 | 0 | 0 |
| 17GL4 × F8-514 | 37 | 10 | 193 | 19.3 | 0 | |||
| 18QR16 | 17GL4 × F6-316 | 26 | 15 | 263 | 17.5 | 188 | 0 | 0 |
| 18QR17 | 17GL4 × F8-325 | 438 | 410 | 6 549 | 16.0 | 470 | 10 | 2.13 |
| 17GL5 × F8-325 | 29 | 8 | 153 | 19.1 | 0 | |||
| 18QR18 | 17GL6 × F9-880 | 37 | 18 | 229 | 12.7 | 188 | 0 | 0 |
| 18QR19 | 17GL7 × F9-1118 | 163 | 156 | 1 657 | 10.6 | 470 | 1 | 0.21 |
| 18QR20 | 17GL7 × F8-325 | 25 | 17 | 327 | 19.2 | 282 | 7 | 2.48 |
| 18QR21 | 17GL8 × F8-325 | 22 | 19 | 384 | 20.2 | 282 | 2 | 0.71 |
| 18QR22 | 17GL8 × F6-316 | 175 | 156 | 2 658 | 17.0 | 470 | 1 | 0.21 |
| 18QR23 | 17GL8 × F8-514 | 44 | 38 | 563 | 14.8 | 470 | 1 | 0.21 |
| 18QR24 | 17GL9 × F8-514 | 274 | 211 | 2 943 | 13.9 | 470 | 1 | 0.21 |
| 18QR25 | 17GL9 × F8-325 | 48 | 11 | 150 | 13.6 | 140 | 1 | 0.71 |
| 18QR26 | 17GL9 × F8-839 | 27 | 10 | 210 | 21.0 | 188 | 10 | 5.32 |
| 18QR27 | 17GL9 × F9-1118 | 175 | 134 | 2 542 | 19.0 | 470 | 1 | 0.21 |
| 18QR28 | 17GL9 × F6-316 | 241 | 229 | 2 987 | 13.0 | 470 | 0 | 0 |
| 18QR29 | 17GL10 × F6-316 | 516 | 505 | 8 583 | 17.0 | 470 | 0 | 0 |
| 18QR30 | 17GL10 × F8-514 | 94 | 86 | 1 530 | 17.8 | 470 | 0 | 0 |
| 18QR31 | 2383 × F6-316 | 294 | 273 | 4 648 | 17.0 | 470 | 1 | 0.21 |
| 18QR32 | 2383 × F8-514 | 582 | 554 | 9 422 | 17.0 | 470 | 0 | 0 |
| 18QR33 | 2383 × F8-839 | 384 | 315 | 4 725 | 15.0 | 470 | 3 | 0.64 |
| 2383 × F9-880 | 29 | 8 | 132 | 16.5 | 0 | |||
| 18QR34 | 2383 × F9-1118 | 234 | 219 | 4 827 | 22.0 | 470 | 1 | 0.21 |
表2 甘蓝BC4代育性恢复材料的结实率和Rfo传递率
Table 2 Seed setting results and TRs of Rfo gene for Rfo restorer in BC4 cabbages
| 新编号 New code | 组合 Combination | 授粉花蕾数 Number of pollinated flowers | 角果数 Number of siliques | 种子数 Number of seeds | 结实率 Seed setting rate | 成苗数 Number of viable plants | Rfo阳性株 Number of Rfo-positive plants | Rfo传递率/% Transmission rate of Rfo in backcross progenies |
|---|---|---|---|---|---|---|---|---|
| 17GL1 × F8-839 | 19 | 10 | 137 | 13.7 | 0 | |||
| 18QR13 | 17GL2 × F9-1118 | 184 | 171 | 2 736 | 16.0 | 470 | 1 | 0.21 |
| 18QR14 | 17GL2 × F8-514 | 39 | 22 | 284 | 12.9 | 0 | ||
| 18QR15 | 17GL3 × F8-514 | 26 | 13 | 239 | 18.4 | 188 | 0 | 0 |
| 17GL4 × F8-514 | 37 | 10 | 193 | 19.3 | 0 | |||
| 18QR16 | 17GL4 × F6-316 | 26 | 15 | 263 | 17.5 | 188 | 0 | 0 |
| 18QR17 | 17GL4 × F8-325 | 438 | 410 | 6 549 | 16.0 | 470 | 10 | 2.13 |
| 17GL5 × F8-325 | 29 | 8 | 153 | 19.1 | 0 | |||
| 18QR18 | 17GL6 × F9-880 | 37 | 18 | 229 | 12.7 | 188 | 0 | 0 |
| 18QR19 | 17GL7 × F9-1118 | 163 | 156 | 1 657 | 10.6 | 470 | 1 | 0.21 |
| 18QR20 | 17GL7 × F8-325 | 25 | 17 | 327 | 19.2 | 282 | 7 | 2.48 |
| 18QR21 | 17GL8 × F8-325 | 22 | 19 | 384 | 20.2 | 282 | 2 | 0.71 |
| 18QR22 | 17GL8 × F6-316 | 175 | 156 | 2 658 | 17.0 | 470 | 1 | 0.21 |
| 18QR23 | 17GL8 × F8-514 | 44 | 38 | 563 | 14.8 | 470 | 1 | 0.21 |
| 18QR24 | 17GL9 × F8-514 | 274 | 211 | 2 943 | 13.9 | 470 | 1 | 0.21 |
| 18QR25 | 17GL9 × F8-325 | 48 | 11 | 150 | 13.6 | 140 | 1 | 0.71 |
| 18QR26 | 17GL9 × F8-839 | 27 | 10 | 210 | 21.0 | 188 | 10 | 5.32 |
| 18QR27 | 17GL9 × F9-1118 | 175 | 134 | 2 542 | 19.0 | 470 | 1 | 0.21 |
| 18QR28 | 17GL9 × F6-316 | 241 | 229 | 2 987 | 13.0 | 470 | 0 | 0 |
| 18QR29 | 17GL10 × F6-316 | 516 | 505 | 8 583 | 17.0 | 470 | 0 | 0 |
| 18QR30 | 17GL10 × F8-514 | 94 | 86 | 1 530 | 17.8 | 470 | 0 | 0 |
| 18QR31 | 2383 × F6-316 | 294 | 273 | 4 648 | 17.0 | 470 | 1 | 0.21 |
| 18QR32 | 2383 × F8-514 | 582 | 554 | 9 422 | 17.0 | 470 | 0 | 0 |
| 18QR33 | 2383 × F8-839 | 384 | 315 | 4 725 | 15.0 | 470 | 3 | 0.64 |
| 2383 × F9-880 | 29 | 8 | 132 | 16.5 | 0 | |||
| 18QR34 | 2383 × F9-1118 | 234 | 219 | 4 827 | 22.0 | 470 | 1 | 0.21 |
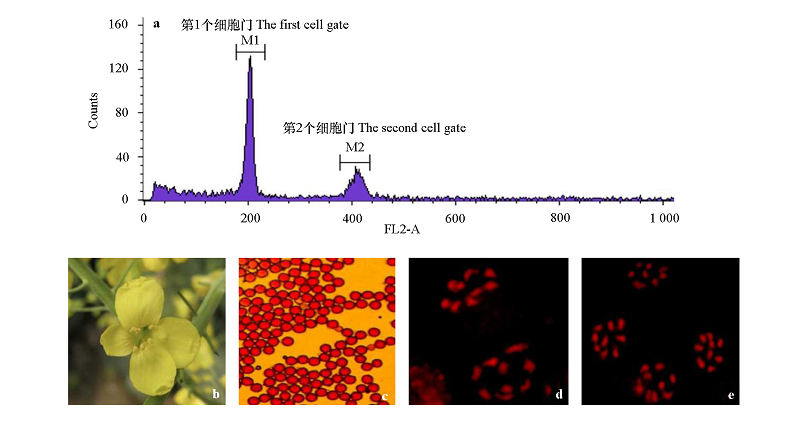
图4 甘蓝BC4代育性恢复系的倍性(a)、育性(b)、花粉活力(c)和染色体配对及分离情况(d、e)
Fig. 4 Ploidy(a),pollen performance(b),pollen viability(c)and chromosome pairing and separation(d,e)of the BC4 Rfo restorer in Brassica oleracea
| 世代 Generation | 编号 Code | 花粉活力/% Pollen viability | Rfo传递率/% Transmission rate of Rfo | 9Ⅱ型染色体配对占比/% Proportion of 9Ⅱ chromosome pairing pattern | 9:9正常染色体 分离占比/% Proportion of normal 9:9 chromosome separation pattern | 倍性荧光峰值 The peaks of G0/G1 peak | 结实率/% Seeds per pod |
|---|---|---|---|---|---|---|---|
| BC4 | 18QR17-216 | 95 | 1.23 | 88 | 90 | 201 | 17.0 |
| 18QR19-426 | 79 | 0.28 | 72 | 66 | 194 | 11.9 | |
| 18QR21-262 | 89 | 0.28 | 84 | 67 | 185 | 13.6 | |
| 18QR33-179 | 75 | 0.56 | 94 | 70 | 189 | 15.2 | |
| 18QR34-410 | 87 | 0.50 | 82 | 52 | 210 | 18.3 | |
| BC5 | 5GH15-169 | 93 | / | 96 | 92 | 197 | / |
| 5GH25-337 | 83 | / | 84 | 88 | 192 | / | |
| 5GH26-234 | 75 | / | 80 | 76 | 199 | / | |
| 5GH26-247 | 77 | / | 74 | 90 | 195 | / | |
| 5GH33-111 | 81 | / | 70 | 96 | 210 | / | |
| 5GH36-589 | 90 | / | 78 | 72 | 218 | / | |
| 5GH39-211 | 91 | / | 82 | 74 | 191 | / |
表3 甘蓝BC4、BC5代重点单株开花结实情况
Table 3 Flowering and seedling of key individuals of BC4 and BC5 generations
| 世代 Generation | 编号 Code | 花粉活力/% Pollen viability | Rfo传递率/% Transmission rate of Rfo | 9Ⅱ型染色体配对占比/% Proportion of 9Ⅱ chromosome pairing pattern | 9:9正常染色体 分离占比/% Proportion of normal 9:9 chromosome separation pattern | 倍性荧光峰值 The peaks of G0/G1 peak | 结实率/% Seeds per pod |
|---|---|---|---|---|---|---|---|
| BC4 | 18QR17-216 | 95 | 1.23 | 88 | 90 | 201 | 17.0 |
| 18QR19-426 | 79 | 0.28 | 72 | 66 | 194 | 11.9 | |
| 18QR21-262 | 89 | 0.28 | 84 | 67 | 185 | 13.6 | |
| 18QR33-179 | 75 | 0.56 | 94 | 70 | 189 | 15.2 | |
| 18QR34-410 | 87 | 0.50 | 82 | 52 | 210 | 18.3 | |
| BC5 | 5GH15-169 | 93 | / | 96 | 92 | 197 | / |
| 5GH25-337 | 83 | / | 84 | 88 | 192 | / | |
| 5GH26-234 | 75 | / | 80 | 76 | 199 | / | |
| 5GH26-247 | 77 | / | 74 | 90 | 195 | / | |
| 5GH33-111 | 81 | / | 70 | 96 | 210 | / | |
| 5GH36-589 | 90 | / | 78 | 72 | 218 | / | |
| 5GH39-211 | 91 | / | 82 | 74 | 191 | / |
| 新编号 New code | 组合 Combination | 授粉花 蕾数 Number of pollinated flowers | 角果数 Number of siliques | 种子数 Number of seeds | 结实率 Seed setting rate | 成苗数 Number of viable plants | Rfo阳性株 Number of Rfo-positive plants | Rfo传递率/% TR of Rfo in backcross progenies |
|---|---|---|---|---|---|---|---|---|
| 5GH1 | J34 × 18QR13-23 | 54 | 45 | 634 | 14.1 | 360 | 0 | |
| 5GH2 | J35 × 18QR17-26 | 69 | 58 | 865 | 14.9 | 288 | 3 | 1.04 |
| 5GH3 | J34 × 18QR17-116 | 104 | 94 | 1 484 | 15.8 | 360 | 3 | 0.83 |
| 5GH4 | J35 × 18QR17-127 | 131 | 102 | 1 536 | 15.1 | 360 | 2 | 0.56 |
| 5GH5 | J34 × 18QR17-175 | 19 | 14 | 290 | 20.7 | 288 | 0 | |
| 5GH6 | J35 × 18QR17-175 | 11 | 4 | 87 | 21.8 | 72 | 1 | 1.39 |
| 5GH7 | J35 × 18QR17-216 | 99 | 87 | 1 474 | 16.9 | 576 | 3 | 0.52 |
| 5GH8 | J35 × 18QR17-253 | 112 | 91 | 1 285 | 14.1 | 576 | 0 | |
| 5GH9 | J35 × 18QR17-303 | 173 | 167 | 2 396 | 14.3 | 360 | 2 | 0.56 |
| 5GH10 | J35 × 18QR17-438 | 94 | 92 | 1 428 | 15.5 | 360 | 1 | 0.28 |
| 5GH11 | J35 × 18QR19-426 | 45 | 39 | 464 | 11.9 | 360 | 1 | 0.28 |
| 5GH12 | J35 × 18QR20-37 | 193 | 183 | 2 945 | 16.1 | 360 | 5 | 1.39 |
| 5GH13 | J34 × 18QR20-73 | 43 | 26 | 420 | 16.2 | 360 | 1 | 0.28 |
| 5GH14 | J34 × 18QR20-93 | 92 | 91 | 1 394 | 15.3 | 360 | 0 | |
| 5GH15 | J35 × 18QR20-155 | 117 | 109 | 2 029 | 18.6 | 360 | 2 | 0.56 |
| 5GH16 | J35 × 18QR20-171 | 65 | 64 | 953 | 14.9 | 360 | 6 | 1.67 |
| 5GH17 | J35 × 18QR20-194 | 348 | 335 | 5 720 | 17.1 | 360 | 1 | 0.28 |
| 5GH18 | J34 × 18QR21-126 | 125 | 117 | 1 901 | 16.2 | 360 | 5 | 1.39 |
| 5GH19 | 2177 × 18QR21-126 | 186 | 176 | 3 292 | 18.7 | 432 | 3 | 0.69 |
| 2183 × 18QR21-126 | 7 | 2 | 28 | 14.0 | 0 | |||
| 5GH21 | J34 × 18QR21-262 | 180 | 278 | 3 793 | 13.6 | 360 | 1 | 0.28 |
| 5GH22 | J34 × 18QR24-311 | 26 | 17 | 330 | 19.4 | 288 | 2 | 0.69 |
| J34 × 18QR25-20 | 8 | 1 | 13 | 13.0 | 0 | |||
| 5GH24 | J35 × 18QR25-20 | 324 | 319 | 5 402 | 16.9 | 432 | 1 | 0.23 |
| 5GH25 | J34 × 18QR26-17 | 338 | 323 | 4 587 | 14.2 | 360 | 7 | 1.94 |
| 5GH26 | J35 × 18QR26-17 | 20 | 19 | 425 | 22.4 | 360 | 8 | 2.22 |
| 5GH27 | J34 × 18QR26-34 | 64 | 56 | 779 | 13.9 | 360 | 0 | |
| 5GH28 | J35 × 18QR26-34 | 72 | 61 | 850 | 13.9 | 360 | 0 | |
| 5GH29 | J34 × 18QR26-104 | 127 | 97 | 1 437 | 14.8 | 360 | 0 | |
| 5GH30 | J35 × 18QR26-104 | 47 | 24 | 485 | 20.2 | 360 | 1 | 0.28 |
| J34 × 18QR26-112 | 38 | 9 | 130 | 14.4 | 0 | |||
| 5GH32 | J34 × 18QR26-135 | 45 | 24 | 338 | 14.1 | 288 | 3 | 1.04 |
| 5GH33 | J34 × 18QR26-136 | 49 | 21 | 361 | 17.2 | 360 | 12 | 3.33 |
| 5GH34 | J35 × 18QR26-188 | 31 | 19 | 352 | 18.5 | 288 | 0 | |
| 5GH35 | J34 × 18QR31-265 | 120 | 82 | 1 389 | 16.9 | 360 | 0 | |
| 5GH36 | J35 × 18QR33-149 | 134 | 69 | 970 | 14.1 | 576 | 7 | 1.22 |
| 5GH37 | J34 × 18QR33-179 | 39 | 37 | 561 | 15.2 | 360 | 2 | 0.56 |
| 5GH38 | J34 × 18QR34-410 | 640 | 633 | 11 399 | 18.0 | 504 | 3 | 0.60 |
| 5GH39 | 2177 × 18QR34-410 | 28 | 25 | 395 | 15.8 | 288 | 1 | 0.35 |
| 5GH40 | 2183 × 18QR34-410 | 132 | 126 | 2 560 | 20.3 | 216 | 1 | 0.46 |
| 5GH41 | 2186 × 18QR17-216 | 244 | 183 | 3 358 | 18.3 | 504 | 4 | 0.79 |
| 5GH42 | 2202 × 18QR17-216 | 125 | 119 | 1 759 | 14.8 | 504 | 2 | 0.40 |
| 5GH43 | 2147 × 18QR17-216 | 183 | 174 | 2 686 | 15.4 | 504 | 22 | 4.37 |
| 5GH44 | 2185 × 18QR17-216 | 133 | 110 | 2 193 | 19.9 | 504 | 1 | 0.20 |
表4 甘蓝BC5代育性恢复材料结实率和Rfo传递率
Table 4 Seed setting results and TRs of Rfo gene for Rfo restorer in BC5 cabbages
| 新编号 New code | 组合 Combination | 授粉花 蕾数 Number of pollinated flowers | 角果数 Number of siliques | 种子数 Number of seeds | 结实率 Seed setting rate | 成苗数 Number of viable plants | Rfo阳性株 Number of Rfo-positive plants | Rfo传递率/% TR of Rfo in backcross progenies |
|---|---|---|---|---|---|---|---|---|
| 5GH1 | J34 × 18QR13-23 | 54 | 45 | 634 | 14.1 | 360 | 0 | |
| 5GH2 | J35 × 18QR17-26 | 69 | 58 | 865 | 14.9 | 288 | 3 | 1.04 |
| 5GH3 | J34 × 18QR17-116 | 104 | 94 | 1 484 | 15.8 | 360 | 3 | 0.83 |
| 5GH4 | J35 × 18QR17-127 | 131 | 102 | 1 536 | 15.1 | 360 | 2 | 0.56 |
| 5GH5 | J34 × 18QR17-175 | 19 | 14 | 290 | 20.7 | 288 | 0 | |
| 5GH6 | J35 × 18QR17-175 | 11 | 4 | 87 | 21.8 | 72 | 1 | 1.39 |
| 5GH7 | J35 × 18QR17-216 | 99 | 87 | 1 474 | 16.9 | 576 | 3 | 0.52 |
| 5GH8 | J35 × 18QR17-253 | 112 | 91 | 1 285 | 14.1 | 576 | 0 | |
| 5GH9 | J35 × 18QR17-303 | 173 | 167 | 2 396 | 14.3 | 360 | 2 | 0.56 |
| 5GH10 | J35 × 18QR17-438 | 94 | 92 | 1 428 | 15.5 | 360 | 1 | 0.28 |
| 5GH11 | J35 × 18QR19-426 | 45 | 39 | 464 | 11.9 | 360 | 1 | 0.28 |
| 5GH12 | J35 × 18QR20-37 | 193 | 183 | 2 945 | 16.1 | 360 | 5 | 1.39 |
| 5GH13 | J34 × 18QR20-73 | 43 | 26 | 420 | 16.2 | 360 | 1 | 0.28 |
| 5GH14 | J34 × 18QR20-93 | 92 | 91 | 1 394 | 15.3 | 360 | 0 | |
| 5GH15 | J35 × 18QR20-155 | 117 | 109 | 2 029 | 18.6 | 360 | 2 | 0.56 |
| 5GH16 | J35 × 18QR20-171 | 65 | 64 | 953 | 14.9 | 360 | 6 | 1.67 |
| 5GH17 | J35 × 18QR20-194 | 348 | 335 | 5 720 | 17.1 | 360 | 1 | 0.28 |
| 5GH18 | J34 × 18QR21-126 | 125 | 117 | 1 901 | 16.2 | 360 | 5 | 1.39 |
| 5GH19 | 2177 × 18QR21-126 | 186 | 176 | 3 292 | 18.7 | 432 | 3 | 0.69 |
| 2183 × 18QR21-126 | 7 | 2 | 28 | 14.0 | 0 | |||
| 5GH21 | J34 × 18QR21-262 | 180 | 278 | 3 793 | 13.6 | 360 | 1 | 0.28 |
| 5GH22 | J34 × 18QR24-311 | 26 | 17 | 330 | 19.4 | 288 | 2 | 0.69 |
| J34 × 18QR25-20 | 8 | 1 | 13 | 13.0 | 0 | |||
| 5GH24 | J35 × 18QR25-20 | 324 | 319 | 5 402 | 16.9 | 432 | 1 | 0.23 |
| 5GH25 | J34 × 18QR26-17 | 338 | 323 | 4 587 | 14.2 | 360 | 7 | 1.94 |
| 5GH26 | J35 × 18QR26-17 | 20 | 19 | 425 | 22.4 | 360 | 8 | 2.22 |
| 5GH27 | J34 × 18QR26-34 | 64 | 56 | 779 | 13.9 | 360 | 0 | |
| 5GH28 | J35 × 18QR26-34 | 72 | 61 | 850 | 13.9 | 360 | 0 | |
| 5GH29 | J34 × 18QR26-104 | 127 | 97 | 1 437 | 14.8 | 360 | 0 | |
| 5GH30 | J35 × 18QR26-104 | 47 | 24 | 485 | 20.2 | 360 | 1 | 0.28 |
| J34 × 18QR26-112 | 38 | 9 | 130 | 14.4 | 0 | |||
| 5GH32 | J34 × 18QR26-135 | 45 | 24 | 338 | 14.1 | 288 | 3 | 1.04 |
| 5GH33 | J34 × 18QR26-136 | 49 | 21 | 361 | 17.2 | 360 | 12 | 3.33 |
| 5GH34 | J35 × 18QR26-188 | 31 | 19 | 352 | 18.5 | 288 | 0 | |
| 5GH35 | J34 × 18QR31-265 | 120 | 82 | 1 389 | 16.9 | 360 | 0 | |
| 5GH36 | J35 × 18QR33-149 | 134 | 69 | 970 | 14.1 | 576 | 7 | 1.22 |
| 5GH37 | J34 × 18QR33-179 | 39 | 37 | 561 | 15.2 | 360 | 2 | 0.56 |
| 5GH38 | J34 × 18QR34-410 | 640 | 633 | 11 399 | 18.0 | 504 | 3 | 0.60 |
| 5GH39 | 2177 × 18QR34-410 | 28 | 25 | 395 | 15.8 | 288 | 1 | 0.35 |
| 5GH40 | 2183 × 18QR34-410 | 132 | 126 | 2 560 | 20.3 | 216 | 1 | 0.46 |
| 5GH41 | 2186 × 18QR17-216 | 244 | 183 | 3 358 | 18.3 | 504 | 4 | 0.79 |
| 5GH42 | 2202 × 18QR17-216 | 125 | 119 | 1 759 | 14.8 | 504 | 2 | 0.40 |
| 5GH43 | 2147 × 18QR17-216 | 183 | 174 | 2 686 | 15.4 | 504 | 22 | 4.37 |
| 5GH44 | 2185 × 18QR17-216 | 133 | 110 | 2 193 | 19.9 | 504 | 1 | 0.20 |
| [1] |
Ayotte R, Harney P M, Machado V S. 1987. The transfer of triazine resistance from Brassica napus L. to B. oleracea L. I. Production of F1hybrids through embryo rescue. Euphytica, 36 (2):615-624.
doi: 10.1007/BF00041511 URL |
| [2] | Bannerot H, Boulidard L, Couderon Y, Temple J. 1974. Transfer of cytoplasmic male sterility from Raphanus sativus to Brassica oleracea//Wills A B,North C. Proc Eucarpia Meet Cruciferae. Scottish Hortic Res Inst,Invergavrie:52-54. |
| [3] |
Brown G G, Formanova N, Jin H, Wargachuk R, Dendy C, Pati P, Lafores M, Zhang J, Cheung W Y, Landry B S. 2003. The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant Journal, 35 (2):262-272.
doi: 10.1046/j.1365-313X.2003.01799.x URL |
| [4] |
Chen R, Chang L, Cai X, Wu J, Liang J L, Lin R M, Song Y, Wang X W. 2020. Development of InDel markers for Brassica rapa based on a high-resolution melting curve. Horticultural Plant Journal, 7 (1):31-37.
doi: 10.1016/j.hpj.2020.05.003 URL |
| [5] |
Delourme R, Eber F. 1992. Linkage between an isozyme marker and a restorer gene in radish cytoplasmic male sterility of rapeseed (Brassica napus L). Theoretical and Applied Genetics, 85 (2-3):222-228.
doi: 10.1007/BF00222863 pmid: 24197308 |
| [6] |
Delourme R, Bouchereau A, Hubert N, Renard M, Landry B S. 1994. Identification of RAPD markers linked to a fertility restorer gene for the Ogura radish cytoplasmic male sterility of rapeseed(Brassica napus L.). Theoretical and Applied Genetics, 88 (6):741-748.
doi: 10.1007/BF01253979 URL |
| [7] |
Delourme R, Foisset N, Horvais R, Barret P, Champagne G, Cheung W Y, Landry B S, Renard M. 1998. Characterisation of the radish introgression carrying the Rfo restorer gene for the Ogu-INRA cytoplasmic male sterility in rapeseed(Brassica napus L.). Theoretical and Applied Genetics, 97 (1-2):129-134.
doi: 10.1007/s001220050876 URL |
| [8] |
Desloire S, Gherbi H, Laloui W, Marhadour S, Clouet V, Cattolico L, Falentin C, Giancola S, Renard M, Budar F, Small I, Caboche M, Delourme R, Bendahmane A. 2003. Identification of the fertility restoration locus Rfo in radish,as a member of the pentatricopeptide-repeat protein family. EMBO Reports, 4 (6):588-594.
pmid: 12740605 |
| [9] |
Duran C, Appleby N, Edwards D, Batley J. 2009. Molecular genetic markers:discovery,applications,data storage and visualization. Current Bioinformatics, 4 (1):16-27.
doi: 10.2174/157489309787158198 URL |
| [10] |
Erali M, Wittwer C T. 2010. High resolution melting analysis for gene scanning. Methods, 50 (4):250-261.
doi: 10.1016/j.ymeth.2010.01.013 URL |
| [11] | Fang Zhiyuan. 2017. Chinese vegetable breeding. Beijing: China Agriculture Press:542-547. (in Chinese) |
| 方智远. 2017. 中国蔬菜育种学. 北京: 中国农业出版社:542-547. | |
| [12] |
Gudi S, Atri C, Goyal A, Kaur N, Akhtar J, Mittal M, Kaur K, Kaur G, Banga S S. 2020. Physical mapping of introgressed chromosome fragment carrying the fertility restoring(Rfo)gene for Ogura CMS in Brassica juncea L. Czern & Coss. Theoretical and Applied Genetics, 133 (10):2949-2959.
doi: 10.1007/s00122-020-03648-3 URL |
| [13] |
Hu X, Mandy S G, Kubik T, Danielson J, Hnatiuk N, Marchione W, Greene T, Thompson S. 2008. Mapping of the Ogura fertility restorer gene Rfo and development of Rfo allele-specific markers in canola(Brassica napus L.). Molecular Breeding, 22 (4):663-674.
doi: 10.1007/s11032-008-9207-1 URL |
| [14] |
Krypuy M, Newnham G M, Thomas D M, Conron M, Dobrovic A. 2006. High resolution melting analysis for the rapid and sensitive detection of mutations in clinical samples:kras codon 12 and 13 mutations in non-small cell lung cancer. BMC Cancer, 6:295.-
doi: 10.1186/1471-2407-6-295 URL |
| [15] | Jiang Jiao, Zhang Enhui, Li Shengjuan, Shi Wenwen, Wu Xiaofeng, Guo Jia, Gong Haijun, Xu Zhongmin. 2019. Obtained interspecific hybrids between Ogura CMS lines of Brassica oleracea and Brassica napus through embryo rescue. Acta Horticulturae Sinica, 46 (3):590-600. (in Chinese) |
| 姜娇, 张恩慧, 李升娟, 石汶汶, 吴晓凤, 郭佳, 宫海军, 许忠民. 2019. 采用胚挽救获得Ogura CMS甘蓝与甘蓝型油菜的种间杂种. 园艺学报, 46 (3):590-600. | |
| [16] |
Li Q F, Mei J Q, Zhang Y J, Li J N, Ge X H, Li Z Y, Qian W. 2013. A large-scale introgression of genomic components of Brassica rapa into B. napus by the bridge of hexaploid derived from hybridization between B. napus and B. oleracea. Theoretical and Applied Genetics, 126 (8):2073-2080.
doi: 10.1007/s00122-013-2119-4 URL |
| [17] | Liu Yuanxia, Lan Jinhao, Bo Suhua, Sun Xiaohong, Liu Chunxiao, Zhang Yugang, Dai Hongyi. 2017. Screening of SNP and InDel markers to glomerella leaf spot resistance gene locus in apple using HRM technology. Acta Horticulturae Sinica, 44 (2):215-222. (in Chinese) |
| 刘源霞, 兰进好, 柏素花, 孙晓红, 刘春晓, 张玉刚, 戴洪义. 2017. 苹果抗炭疽菌叶枯病基因SNP和InDel标记的HRM筛选. 园艺学报, 44 (2):215-222. | |
| [18] | Luo Wenlong, Guo Tao, Zhou Danhua, Chen Haiying, Wang Hui, Chen Zhiqiang, Liu Yongzhu. 2013. Analysis of rice genotypes rice Wx and fgr by HRM-based functional marker. Journal of Hunan Agricultural University(Natural Sciences), 39 (6):597-603. (in Chinese) |
| 罗文龙, 郭涛, 周丹华, 陈海英, 王慧, 陈志强, 刘永柱. 2013. 利用基于HRM的功能标记分析水稻Wx和fgr的基因型. 湖南农业大学(自然科学版), 39 (6):597-603. | |
| [19] |
Mei J Q, Liu Y, Wei D Y, Wittkop B, Ding Y J, Li Q F, Li J N, Wan H F, Li Z Y, Ge X H, Frauen W. 2015. Transfer of sclerotinia resistance from wild relative of Brassica oleracea into Brassica napus using a hexaploidy step. Theoretical and Applied Genetics, 128 (4):639-644.
doi: 10.1007/s00122-015-2459-3 URL |
| [20] |
Palais R, Zhou L, Montgomery J, Wittwer C T. 2007. Simultaneous mutation scanning and genotyping by high-resolution DNA melting analysis. Nature Protocols, 2 (1):59-66.
pmid: 17401339 |
| [21] | Pearson O H. 1972. Cytoplasmically inherited male sterility characters and flavor components from the species cross Brassica nigra(L.)Koch × B. oleracea L. Amer Soc Hort Sci J, 97 (3):397-402. |
| [22] | Ren W J, Li Z Y, Han F Q, Zhang B, Li X, Fang Z Y, Yang L M, Zhuang M, Lv H H, Liu Y M, Wang Y, Yu H L, Zhang Y Y. 2020. Utilization of Ogura CMS germplasm with the clubroot resistance gene by fertility restoration and cytoplasm replacement in Brassica oleracea L. Horticculture Research, 7:61. |
| [23] |
Ripley V L, Beversdorf W D. 2003. Development of self-incompatible Brassica napus(I)introgression of S-alleles from Brassica oleracea through interspecific hybridization. Plant Breeding, 122 (1):1-5.
doi: 10.1046/j.1439-0523.2003.00780.x URL |
| [24] | Saghai-Maroof M A. 1984. Ribosomal DNA spacer-length polymorphisms in barley:Mendelian inheritance,chromosomal location and population dynamics. Proceedings of the National Academy of Sciences of the United States of America, 81 (24):8014-8018. |
| [25] |
Simko I. 2016. High-Resolution DNA melting analysis in plant research. Trends in Plant Science, 21 (6):528-537.
doi: S1360-1385(16)00005-4 pmid: 26827247 |
| [26] | Su Xiaomei. 2014. The development and application of tomato foreground and background markers[M. D. Dissertation]. Beijing: Chinese Academy of Agricultural Sciences. (in Chinese) |
| 苏晓梅. 2014. 番茄前景标记和背景标记的开发与应用研究[硕士论文]. 北京: 中国农业科学院. | |
| [27] |
Thompson K F. 1972. Cytoplasmic male sterility in oilseed rape. Heredity, 29 (2):253-257.
doi: 10.1038/hdy.1972.89 URL |
| [28] |
Tan Y Y, Fu H W, Zhao H J, Lu S, Fu J J, Li Y F, Cui H R, Shu Q Y. 2013. Functional molecular markers and high-resolution melting curve analysis of low phytic acid mutations for marker-assisted selection in rice. Molecular Breeding, 31 (3):517-528.
doi: 10.1007/s11032-012-9809-5 URL |
| [29] |
Tan Y Y, Yu X M, Shu Q Y, Zhang H L, Wang S G, Yuan F J, Shi C H. 2016. Development of an HRM-based,safe and high-throughput genotyping system for two low phytic acid mutations in soybean. Molecular Breeding, 36 (7):1-9.
doi: 10.1007/s11032-015-0425-z URL |
| [30] | Wang Qingbiao, Zhang Li, Wen Changlong, Yang Jingjing. 2017. Development and application of high-throughput SNP markers for Ogura-CMS fertility restorer gene in radish. Acta Horticulturae Sinica, 44 (7):1309-1318. (in Chinese) |
| 王庆彪, 张丽, 温常龙, 杨静静. 2017. 萝卜Ogura-CMS育性恢复基因Rfo的高通量 SNP 标记开发及其应用. 园艺学报, 44 (7):1309-1318. | |
| [31] |
Wittwer C T, Reed G H, Gundry C N, Vandersteen J G, Pryor R J. 2003. High-resolution genotyping by amplicon melting analysis using LC-Green. Clinical Chemistry, 49 (6):853-860.
doi: 10.1373/49.6.853 URL |
| [32] | Yang Limei, Fang Zhiyuan, Zhuang Mu, Zhang Yangyong, Lü Honghao, Liu Yumei, Li Zhansheng. 2016. Advances of Research on Cabbage Genetics and Breeding during‘The Twelfth Five-year Plan’in China. China Vegetables,(11):1-6. (in Chinese) |
| 杨丽梅, 方智远, 庄木, 张扬勇, 吕红豪, 刘玉梅, 李占省. 2016. “十二五”我国甘蓝遗传育种研究进展. 中国蔬菜,(11):1-6. | |
| [33] |
Yarrow S A, Burnett L A, Wildeman R P, Kemble R J. 1990. The transfer of‘Polima’cytoplasmic male sterility from oilseed rape(Brassica napus)to broccoli(B. oleracea)by protoplast fusion. Plant Cell Reports, 9 (4):185-188.
doi: 10.1007/BF00232176 pmid: 24226699 |
| [34] | Yu Hailong. 2015. Creation of Ogura-CMS fertility-restored materials in Brassica oleracea through distant hybridization[M. D. Dissertation]. Beijing: Chinese Academy of Agricultural Sciences. (in Chinese) |
| 于海龙. 2015. 通过远缘杂交创制甘蓝Ogura细胞质雄性不育的恢复材料[硕士论文]. 北京: 中国农业科学院. | |
| [35] | Yu Hailong. 2018. Creation of Ogura-CMS fertility-restored materials in Brassica oleracea through distant hybridization[M. D. Dissertation]. Yangling: Northwest A & F University. (in Chinese) |
| 于海龙. 2018. 甘蓝类蔬菜Ogura CMS恢复材料的创制及其遗传构成分析[博士论文]. 杨凌: 西北农林科技大学. | |
| [36] |
Yu H L, Fang Z Y, Liu Y M, Yang L M, Zhuang M, Lv H H, Li Z S, Han F Q, Liu X P, Zhang YY. 2016. Development of a novel allele-specific Rfo marker and creation of Ogura CMS fertility-restored interspecific hybrids in Brassica oleracea. Theoretical and Applied Genetics, 129 (8):1625-1637.
doi: 10.1007/s00122-016-2728-9 URL |
| [37] |
Yu H L, Li Z Y, Yang L M, Liu Y M, Zhuang M, Zhang L G, Lv H H, Li Z S, Han F Q, Liu X P, Fang Z Y, Zhang Y Y. 2017. Morphological and molecular characterization of the second backcross progenies of Ogu-CMS Chinese kale and rapeseed. Euphytica, 213 (2):55.
doi: 10.1007/s10681-017-1842-3 URL |
| [38] |
Yu H L, Li Z Y, Ren W J, Han F Q, Yang L M, Zhuang M, Lv H H, Liu Y M, Fang Z Y, Zhang Y Y. 2020. Creation of fertility-restored materials for Ogura CMS in Brassica oleracea by introducing Rfo gene from Brassica napus via an allotriploid strategy. Theoretical and Applied Genetics, 133:2825-2837.
doi: 10.1007/s00122-020-03635-8 URL |
| [39] | Yu Hailong, Ren Wenjing, Fang Zhiyuan, Yang Limei, Zhuang Mu, Lü Honghao, Wang Yong, Ji Jialei, Zhang Yangyong. 2021. Advances on fertility restoration for cytoplasmic male sterility in vegetables. Acta Horticulturae Sinica, 48 (5):1031-1046. (in Chinese) |
| 于海龙, 任文静, 方智远, 杨丽梅, 庄木, 吕红豪, 王勇, 季佳磊, 张扬勇. 2021. 蔬菜细胞质雄性不育的育性恢复研究进展. 园艺学报, 48 (5):1031-1046. | |
| [40] | Zhang Li, Wang Qingbiao, Wang Yanping. 2020. Distribution of orf687,a fertility restorer gene for Ogura CMS in radish. Acta Horticulturae Sinica, 47 (5):864-874. (in Chinese) |
| 张丽, 王庆彪, 王艳萍. 2020. Ogura CMS育性恢复基因orf687在萝卜中的分布. 园艺学报, 47 (5):864-874. | |
| [41] |
Zhang Y Y, Fang Z Y, Wang Q B, Liu Y M, Yang L M, Zhuang M, Sun P T. 2012. Chloroplast Subspecies-specific SNP detection and its maternal inheritance in Brassica oleracea L. by using a dCAPS marker. Journal of Heredity, 103 (4):606-611.
doi: 10.1093/jhered/ess006 URL |
| [42] | Zhang Zhigang, Si Liying, Zhao Zhizhong, Wang Ronghua, Wang Lihua, Li Qiaoyun, Liu Shuantao. 2018. Construction and usage of high efficient InDel marker detecting method of Chinese cabbage based on high-resolution melting(HRM). Shandong Agricultural Sciences, 50 (10):134-139. (in Chinese) |
| 张志刚, 司立英, 赵智中, 王荣花, 王立华, 李巧云, 刘栓桃. 2018. 基于HRM技术的大白菜InDel标记高效检测技术构建及其应用. 山东农业科学, 50 (10):134-139. | |
| [45] | Zhang Zhigang, Zhao Zhizhong, Li Qiaoyun, Wang Xiao, Liu Shuantao, Wang Shufen, Xu Wenling, Liu Xianxian, Liu Chen. 2016. Development of InDels markers and their usage in detection of residual heterozygous lines in Chinese cabbage(Brassica rapa ssp. pekinensis). Journal of Agricultural Biotechnology, 24 (4):510-518. (in Chinese) |
| 张志刚, 赵智中, 李巧云, 王晓, 刘栓桃, 王淑芬, 徐文玲, 刘贤娴, 刘辰. 2016. 大白菜InDels标记开发及其在剩余杂合体鉴定中的应用. 农业生物技术学报, 24 (4):510-518. | |
| [46] | Zhu Yanfang, Zhang Wei, Hu Jin, Zhu Liwei, Guan Yajing, Wang Jiancheng. 2013. High resolution melting curve analysis and its application on identification of plant germplasm. Seeds, 32 (10):57-60. (in Chinese) |
| 朱岩芳, 张炜, 胡晋, 朱丽伟, 关亚静, 王建成. 2013. 高分辨率熔解曲线分析(HRM)及其在植物种质资源鉴定中的应用. 种子, 32 (10):57-60. |
| [1] | 韩 睿, 钟雄辉, 陈登辉, 崔 建, 乐祥庆, 颉建明, 康俊根, . 黑腐病菌效应因子XopR的甘蓝靶标基因BobHLH34的克隆及功能分析[J]. 园艺学报, 2023, 50(2): 319-330. |
| [2] | 崔建, 钟雄辉, 刘泽慈, 陈登辉, 李海龙, 韩睿, 乐祥庆, 康俊根, 王超. 结球甘蓝染色体片段替换系构建[J]. 园艺学报, 2023, 50(1): 65-78. |
| [3] | 何成勇, 赵晓丽, 许腾飞, 高德航, 李世访, 王红清. 草莓病毒1山东分离物全基因组分析[J]. 园艺学报, 2023, 50(1): 153-160. |
| [4] | 吕红豪, 杨丽梅, 方智远, 张扬勇, 庄 木, 刘玉梅, 王 勇, 季家磊, 李占省, 韩风庆. 春甘蓝新品种‘中甘27’和‘中甘28’[J]. 园艺学报, 2022, 49(S1): 63-64. |
| [5] | 周 娜, 陶伟林, 陆景伟, 郑 阳, 胡 燕, 潘晓雪. 甘蓝新品种‘渝园春印’[J]. 园艺学报, 2022, 49(S1): 65-66. |
| [6] | 王勇健, 孔俊花, 范培格, 梁振昌, 金秀良, 刘布春, 代占武. 葡萄表型组高通量获取及分析方法研究进展[J]. 园艺学报, 2022, 49(8): 1815-1832. |
| [7] | 李强, 蔡玉梅, 苏彦宾, 王英, 顾丽嫱, 赵玉倩. 中早熟甘蓝新品种‘劲绿60’[J]. 园艺学报, 2022, 49(8): 1835-1836. |
| [8] | 陈道宗, 刘镒, 沈文杰, 朱博, 谭晨. 白菜、甘蓝和甘蓝型油菜PAP1/2同源基因的鉴定及分析[J]. 园艺学报, 2022, 49(6): 1301-1312. |
| [9] | 黄静静, 刘伟, 刘玉梅, 韩风庆, 方智远, 杨丽梅, 庄木, 张扬勇, 吕红豪, 王勇, 季家磊, 李占省. Ogura CMS青花菜育性恢复材料创制及其遗传背景研究[J]. 园艺学报, 2022, 49(3): 533-547. |
| [10] | 杨丽梅, 方智远. 中国甘蓝遗传育种研究60年[J]. 园艺学报, 2022, 49(10): 2075-2098. |
| [11] | 吕红豪, 杨丽梅, 方智远, 张扬勇, 庄木, 刘玉梅, 王勇, 季家磊, 李占省, 韩风庆. 春甘蓝新品种'中甘26'[J]. 园艺学报, 2022, 49(10): 2285-2286. |
| [12] | 吕红豪, 杨丽梅, 方智远, 张扬勇, 庄木, 刘玉梅, 孙培田, 王勇, 季家磊, 李占省, 韩风庆. 抗枯萎病早熟优质春甘蓝新品种‘YR中甘21’[J]. 园艺学报, 2021, 48(9): 1839-1840. |
| [13] | 张贺翠, 王玉奎, 左同鸿, 刘倩莹, 张以忠, 胡燈科, 谢琴琴, 朱利泉. 甘蓝BoPID基因的克隆与分析[J]. 园艺学报, 2021, 48(6): 1123-1134. |
| [14] | 董艺, 冯羽飞, 许忠民, 王世民, 唐鸿吕, 黄炜. SSR标记遗传距离与结球甘蓝杂种优势的关系分析[J]. 园艺学报, 2021, 48(5): 934-946. |
| [15] | 卜方迪, 陈俊光, 刘贞, 向本春, 申冕, 崔百明, 郑银英. 新疆蟠桃中发现油桃茎痘相关病毒和亚洲李属病毒[J]. 园艺学报, 2021, 48(1): 49-59. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司