
园艺学报 ›› 2021, Vol. 48 ›› Issue (8): 1552-1564.doi: 10.16420/j.issn.0513-353x.2020-0987
褚洒洒1, 范祥祯2, 邱肇凯1, 杨琪1, 张毓婷1, 王洋1, 徐晓珊1, 童再康1, 张俊红1,*( )
)
收稿日期:2021-04-19
修回日期:2021-06-21
出版日期:2021-08-25
发布日期:2021-09-06
通讯作者:
张俊红
E-mail:zhangjunhong@zafu.edu.cn
基金资助:
CHU Sasa1, FAN Xiangzhen2, QIU Zhaokai1, YANG Qi1, ZHANG Yuting1, WANG Yang1, XU Xiaoshan1, TONG Zaikang1, ZHANG Junhong1,*( )
)
Received:2021-04-19
Revised:2021-06-21
Online:2021-08-25
Published:2021-09-06
Contact:
ZHANG Junhong
E-mail:zhangjunhong@zafu.edu.cn
摘要:
采用顶空—固相微萃取和气相色谱—质谱联用技术(HS-SPME-GC-MS)测定浙江楠(Phoebe chekiangensis)、闽楠(P. bournei)、紫楠(P. sheareri)、小叶桢楠(P. zhennan f. oblong)和大叶桢楠(P. zhennan f. elliptical)叶片挥发性成分,并比较浙江楠叶片在不同季节、不同方位和不同温度下的挥发性成分差异及其日变化规律。结果表明,5种楠属植物叶片共检测出80种化合物,其中浙江楠52种、闽楠55种、紫楠41种、小叶桢楠56种、大叶桢楠46种,(+)-β-瑟林烯、3-己烯-1-醇和β-榄香烯可能为浙江楠叶片香气的特征物质。在夏季和秋季浙江楠叶片中分别鉴定出70和64种化合物,其中夏季西方位叶片成分种类最多,而秋季北方位叶片成分种类最多。正交偏最小二乘法判别分析(OPLS-DA)表明南、北方位叶片成分差异大,而东、西方位叶片成分较相似。α-古巴烯和α-葎草烯等含量在不同温度培养下的浙江楠叶片中存在显著差异,其中15和25 ℃下挥发性成分相近,而45 ℃下的成分显著区别于其他温度。挥发性成分日变化分析发现浙江楠叶片α-古巴烯、β-荜澄茄烯和γ-摩勒烯等8种物质在12:00或14:00出现高峰,而β-石竹烯、右旋大根香叶烯和β-榄香烯等10种物质在12:00或14:00最低。研究结果可为浙江楠等楠属植物精油的开发利用及以楠属植物为主体林分的森林康养提供参考。
中图分类号:
褚洒洒, 范祥祯, 邱肇凯, 杨琪, 张毓婷, 王洋, 徐晓珊, 童再康, 张俊红. 楠属5种植物叶片的挥发性成分的测定[J]. 园艺学报, 2021, 48(8): 1552-1564.
CHU Sasa, FAN Xiangzhen, QIU Zhaokai, YANG Qi, ZHANG Yuting, WANG Yang, XU Xiaoshan, TONG Zaikang, ZHANG Junhong. Determination of Leaf Volatile Components from Five Phoebe Species[J]. Acta Horticulturae Sinica, 2021, 48(8): 1552-1564.
| 类别 Class | 编号 No. | 保留指数 Retention index | 化合物 Compounds | 相对含量/% Relative content | ||||
|---|---|---|---|---|---|---|---|---|
| 浙江楠 P. chekian- gensis | 小叶桢楠 P. zhennan f. oblong | 大叶桢楠 P. zhennan f. elliptical | 紫楠 P. sheareri | 闽楠 P. bour- nei | ||||
| 萜烯类(单萜) | A1 | 933 | 蒎烯Pinene | 3.37 | 1.44 | 0.88 | 8.25 | 0.44 |
| Terpenes | A2 | 943 | α-蒎烯α-Pinene | 0.32 | 0.21 | 0.43 | 0.82 | 5.09 |
| (monoterpene) | A3 | 981 | β-蒎烯β-Pinene | – | – | – | – | 3.57 |
| A4 | 949 | 莰烯Camphene | 0.71 | 0.63 | 0.27 | 0.97 | 1.16 | |
| A5 | 977 | (-)-β-蒎烯 (-)-β-Pinene | 1.48 | 0.62 | 0.45 | 5.82 | 0.27 | |
| A6 | 997 | 月桂烯 Myrcene | 0.27 | 0.27 | – | 0.57 | 0.54 | |
| A7 | 1 007 | 水芹烯 Phellandrene | 0.18 | 0.36 | – | – | 0.16 | |
| A8 | 1 024 | S-(-)-柠檬烯 S-(-)-Limonene | – | – | – | – | 0.40 | |
| A9 | 1 057 | (+)-柠檬烯 (+)-Limonene | 1.11 | 0.43 | 0.13 | 1.85 | – | |
| A10 | 1 044 | (E)-β-罗勒烯 (E)-β-Ocimene | 0.18 | 1.66 | – | – | 1.14 | |
| A11 | 1 054 | (Z)-13,7-dimethyl-3,6-octatriene | 0.20 | 1.59 | 0.37 | 0.45 | 1.46 | |
| A12 | 1 093 | 萜品油烯 Terpinolene | – | 0.11 | – | 0.12 | – | |
| A13 | 1 134 | 别罗勒烯(E,Z)-2,6- Dimethylocta-2,4,6-triene | 0.19 | 1.54 | 0.35 | 0.81 | 0.61 | |
| 萜烯类(倍半萜) Terpenes (sesquiterpene) | A14 | 1 331 | 1-ethenyl-1-methyl-4-propan-2-ylidene-2-prop-1-en-2-ylcyclohexane | 5.60 | 6.16 | 6.50 | 0.47 | 6.74 |
| A15 | 1 344 | (-)-α-荜澄茄油烯 (-)-α-Cubebene | 1.54 | 5.60 | 7.00 | 0.61 | 2.55 | |
| A16 | 1 360 | (+)-长叶环烯 (+)-Longicyclene | – | – | – | – | 0.38 | |
| A17 | 1 363 | (+)-环苜蓿烯 (+)-Cyclosativene | – | 0.41 | 0.60 | – | – | |
| A18 | 1 370 | 1,2,4a,5,6,8a-hexahydro-1-isopropyl-4,7-dimethylnaphthalene | – | – | – | – | 0.15 | |
| A19 | 1 376 | α-古巴烯 α-Copaene | 7.35 | 19.05 | 22.94 | 2.48 | 12.66 | |
| A20 | 1 384 | β-波旁烯 β-Bourbonene | 1.40 | 2.11 | 3.32 | 0.41 | 2.48 | |
| A21 | 1 390 | β-荜澄茄油烯 β-Cubebene | 3.34 | 8.29 | 3.85 | 8.80 | 5.13 | |
| A22 | 1 393 | β-榄香烯β-Elemene | 6.84 | 2.79 | 2.10 | 6.40 | 4.02 | |
| A23 | 1 407 | 2,6-二甲基-6-(4-甲基- 3-戊烯基) 2,6-Dimethyl-6-(4-methyl-3-pentenyl)bicyclo[3.1.1]hept-2-ene | 0.65 | 0.52 | 0.24 | 0.29 | – | |
| A24 | 1 411 | (-)-α-古芸烯 (-)-α-Gurjunene | 0.35 | 1.64 | 1.09 | 0.64 | 1.31 | |
| A25 | 1 418 | (1S,5S)-4,6-二甲基-6- (4-甲基戊-3-烯基)双环 [3.1.1]庚-3-烯 (1S,5S)-4,6-Dimethyl-6-(4-methylpent-3-enyl)bicyclo[3.1.1]hept-3-ene | 0.65 | 0.29 | 0.35 | 3.28 | – | |
| A26 | 1 422 | β-石竹烯β-Caryophyllene | 8.01 | 3.33 | 5.58 | 10.42 | 5.70 | |
| A27 | 1 448 | 马兜铃烯Aristolene | 2.14 | 1.47 | 2.84 | 0.17 | 2.14 | |
| A28 | 1 454 | γ-广藿香烯γ-Patchoulene | 1.68 | 1.06 | 2.32 | – | 1.58 | |
| A29 | 1 459 | α-葎草烯α-Humulene | 1.87 | 2.91 | 2.48 | 24.41 | 3.90 | |
| A30 | 1 463 | β-倍半水芹烯 β-Sesquiphellandrene | 0.79 | 0.11 | – | – | – | |
| A31 | 1 466 | 香树烯(-)-Alloaromadendrene | 0.57 | 1.62 | 2.06 | – | 1.02 | |
| A32 | 1 475 | α-Himachalene | – | – | – | – | 0.53 | |
| A33 | 1 494 | (+)-β-瑟林烯 (+)-β-Selinene | 3.76 | 2.67 | 0.54 | 1.87 | 3.01 | |
| A34 | 1 489 | 右旋大根香叶烯 (+)- Germacrene D | 6.50 | 3.70 | 9.41 | 2.29 | 6.13 | |
| A35 | 1 498 | 愈创木烯Guaiacene | 3.09 | 1.58 | 0.14 | 0.70 | 1.19 | |
| A36 | 1 413 | (+)-γ-古芸烯 (+)-γ-Gurjunene | – | – | – | – | 3.71 | |
| A37 | 1 428 | (-)-β-花柏烯 (-)-β-Chamigrene | – | – | – | – | 2.50 | |
| A38 | 1 435 | γ-榄香烯γ-Elemene | – | – | – | – | 0.67 | |
| A39 | 1 505 | 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-cyclohexane | 5.09 | 7.03 | 6.42 | 0.42 | 6.43 | |
| A40 | 1 510 | (+)-香橙烯 (+)-Aromadendrene | 0.66 | 0.67 | 1.00 | 0.13 | – | |
| 类别 Class | 编号 No. | 保留指数 Retention index | 化合物 Compounds | 相对含量/% Relative content | ||||
| 浙江楠 P. chekian- gensis | 小叶桢楠 P. zhennan f. oblong | 大叶桢楠 P. zhennan f. elliptical | 紫楠 P. sheareri | 闽楠 P. bour- nei | ||||
| A41 | 1 490 | -Azulene,1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethylidene)-, (1S,4S)- | – | 0.13 | – | 0.70 | – | |
| A42 | 1 523 | (+)-γ-荜澄茄烯 (+)-γ-Cadinene | – | – | – | – | 0.53 | |
| A43 | 1 533 | δ-荜澄茄烯 δ-Cadinene | 6.01 | 1.87 | 3.57 | 1.12 | 1.81 | |
| A44 | 1 609 | α-广藿香烯 α-Patchoulene | 0.13 | 0.41 | 0.11 | 0.25 | 0.42 | |
| A45 | 1 621 | 环氧化蛇麻烯Ⅱ Humulene1,2-epoxide | 0.18 | 0.27 | 0.14 | 0.55 | – | |
| 醛类 Aldehydes | B1 | 1 645 | 2-甲基-4-戊醛 2-Methylpent-4-enal | 1.49 | 0.69 | 0.25 | 0.10 | 0.51 |
| B2 | 1 124 | 壬醛 Nonanal | 0.13 | – | – | – | 0.12 | |
| B3 | 1 210 | 癸醛 Decanal | 0.17 | 0.35 | 0.27 | – | – | |
| B4 | 1 305 | 10-十一烯醛10-Undecenal | – | – | – | – | 0.13 | |
| B5 | 1 310 | 十一醛 Undecanal | – | 0.33 | – | – | 0.13 | |
| B6 | 1 412 | 十二醛 Lauryl aldehyde | 0.16 | 1.02 | 0.13 | – | – | |
| B7 | 1 520 | 十四烷醛 Tetradecanal | – | – | – | 0.17 | – | |
| 醇类 Alcohols | C1 | 2 510 | 反式-3-己烯-1-醇 Trans-3-Hexen-1-ol | 2.08 | 1.57 | 1.02 | 1.51 | 1.50 |
| C2 | 2 738 | 3-甲基-4-戊醇 3-Methylpent-4-en-1-ol | 0.22 | 0.43 | – | 0.28 | – | |
| C3 | 1 409 | 环十二醇Cyclododecanol | – | – | – | – | 0.39 | |
| C4 | 2 714 | 叶醇cis-3-Hexen-1-ol | 0.17 | – | 0.36 | – | – | |
| 萜烯醇类 | D1 | 1 035 | 桉叶油醇 Cineole | 0.10 | – | – | 3.97 | – |
| Terpene alcohols | D2 | 1 108 | 芳樟醇 Linalool | 0.43 | – | – | – | – |
| D3 | 1 094 | 橙花醇 Nerol | – | – | – | – | 0.14 | |
| D4 | 1 295 | 紫苏醇 Perillyl alcohol | – | – | – | – | 0.13 | |
| D5 | 1 540 | α-檀香醇 α-Santalol | – | 0.13 | 0.11 | – | – | |
| D6 | 1 526 | α-人参烯 α-Panasinsanene | 0.11 | – | – | – | – | |
| D7 | 1 584 | 4(15),5,10(14)-大根香叶三 烯-1-醇 4(15),5,10(14)-Germacratrien-1-ol | – | 0.14 | 0.13 | – | – | |
| D8 | 1 590 | 桉油烯醇 Spathulenol | – | 0.68 | 0.22 | – | 0.47 | |
| D9 | 1 633 | Β-桉叶醇 β-Eudesmol | 0.16 | 0.10 | – | – | – | |
| D10 | 1 650 | 1aS,4aS,7R,7aS,7bS)-1,1,7- Trimethyl-4-methylidene-1a,2,3, 4a,5,6,7a,7b-octahydrocyclopropa [h]azulen-7-ol | – | 0.11 | – | – | – | |
| 酯类 Esters | E1 | 1 027 | (E)-3-己烯-1-醇乙酸酯 Trans-3-hexenyl acetate | 0.26 | 0.21 | 0.54 | 0.49 | 3.57 |
| E2 | 1 028 | 乙酸叶醇酯 cis-3-Hexenyl acetate | – | 0.10 | – | – | – | |
| 萜烯酯类 | F1 | 1 265 | 乙酸芳樟酯 Linalyl acetate | 0.23 | 0.41 | 0.17 | – | 0.91 |
| Terpene esters | F2 | 1 299 | 乙酸龙脑酯 Bornyl acetate | 3.70 | 0.98 | 0.62 | 5.10 | – |
| F3 | 1 292 | 左旋乙酸冰片酯 L-Bornyl acetate | – | – | – | – | 2.24 | |
| 萘类 Naphthalenes | G1 | 1 517 | (1aR)-1aβ,2,3,3a,4,5,6,7bβ- Octahydro-1,1,3aβ,7-tetramethyl- 1H-cyclopropa[a]naphthalene | 1.85 | 0.89 | 1.59 | 0.35 | – |
| G2 | 1 542 | 1,2,3,4,4a,7-Hexahydro-1,6- dimethyl-4-(1-methylethyl)- naphthalene | 0.35 | 0.30 | 0.29 | – | 0.20 | |
| G3 | 1 548 | 1,2,4a,5,6,8a-Hexahydro-4,7- dimethyl-1-(1-methylethyl) napthtalene | 0.15 | 0.14 | 0.24 | 0.11 | 0.41 | |
| G4 | 1 578 | (8aα)-Decahydro-1α-isopropyl- 3aα-methyl-7-methylene-4α,8α- epoxyazulene | – | 0.19 | 0.10 | – | 0.42 | |
| 类别 Class | 编号 No. | 保留指数 Retention index | 化合物 Compounds | 相对含量/% Relative content | ||||
| 浙江楠 P. chekian- gensis | 小叶桢楠 P. zhennan f. oblong | 大叶桢楠 P. zhennan f. elliptical | 紫楠 P. sheareri | 闽楠 P. bour- nei | ||||
| 烷烃类 Alkanes | H1 | 1 303 | 正十三烷Tridecane | – | – | – | 0.10 | 0.13 |
| H2 | 1 308 | 环己烷Cyclohexane | – | – | – | – | 0.67 | |
| H3 | 1 556 | (E)-2-(6,10-Timethylundeca-1,5,9- trien-2-yl)oxirane | 0.11 | – | – | – | – | |
| 有机酸 Organic acid | I1 | 1 283 | (1R,4S)-1,7,7-Trimethylbicyclo [2.2.1]heptan-2-yl acetate | – | – | – | – | 0.33 |
| 氧化物 Oxide | J1 | 1 595 | 石竹素Caryophyllene oxide | 0.49 | 0.34 | 0.22 | 0.21 | 0.36 |
表1 楠属5种植物的叶片挥发性成分分析
Table 1 Analysis of volatile components in leaves of five Phoebe species
| 类别 Class | 编号 No. | 保留指数 Retention index | 化合物 Compounds | 相对含量/% Relative content | ||||
|---|---|---|---|---|---|---|---|---|
| 浙江楠 P. chekian- gensis | 小叶桢楠 P. zhennan f. oblong | 大叶桢楠 P. zhennan f. elliptical | 紫楠 P. sheareri | 闽楠 P. bour- nei | ||||
| 萜烯类(单萜) | A1 | 933 | 蒎烯Pinene | 3.37 | 1.44 | 0.88 | 8.25 | 0.44 |
| Terpenes | A2 | 943 | α-蒎烯α-Pinene | 0.32 | 0.21 | 0.43 | 0.82 | 5.09 |
| (monoterpene) | A3 | 981 | β-蒎烯β-Pinene | – | – | – | – | 3.57 |
| A4 | 949 | 莰烯Camphene | 0.71 | 0.63 | 0.27 | 0.97 | 1.16 | |
| A5 | 977 | (-)-β-蒎烯 (-)-β-Pinene | 1.48 | 0.62 | 0.45 | 5.82 | 0.27 | |
| A6 | 997 | 月桂烯 Myrcene | 0.27 | 0.27 | – | 0.57 | 0.54 | |
| A7 | 1 007 | 水芹烯 Phellandrene | 0.18 | 0.36 | – | – | 0.16 | |
| A8 | 1 024 | S-(-)-柠檬烯 S-(-)-Limonene | – | – | – | – | 0.40 | |
| A9 | 1 057 | (+)-柠檬烯 (+)-Limonene | 1.11 | 0.43 | 0.13 | 1.85 | – | |
| A10 | 1 044 | (E)-β-罗勒烯 (E)-β-Ocimene | 0.18 | 1.66 | – | – | 1.14 | |
| A11 | 1 054 | (Z)-13,7-dimethyl-3,6-octatriene | 0.20 | 1.59 | 0.37 | 0.45 | 1.46 | |
| A12 | 1 093 | 萜品油烯 Terpinolene | – | 0.11 | – | 0.12 | – | |
| A13 | 1 134 | 别罗勒烯(E,Z)-2,6- Dimethylocta-2,4,6-triene | 0.19 | 1.54 | 0.35 | 0.81 | 0.61 | |
| 萜烯类(倍半萜) Terpenes (sesquiterpene) | A14 | 1 331 | 1-ethenyl-1-methyl-4-propan-2-ylidene-2-prop-1-en-2-ylcyclohexane | 5.60 | 6.16 | 6.50 | 0.47 | 6.74 |
| A15 | 1 344 | (-)-α-荜澄茄油烯 (-)-α-Cubebene | 1.54 | 5.60 | 7.00 | 0.61 | 2.55 | |
| A16 | 1 360 | (+)-长叶环烯 (+)-Longicyclene | – | – | – | – | 0.38 | |
| A17 | 1 363 | (+)-环苜蓿烯 (+)-Cyclosativene | – | 0.41 | 0.60 | – | – | |
| A18 | 1 370 | 1,2,4a,5,6,8a-hexahydro-1-isopropyl-4,7-dimethylnaphthalene | – | – | – | – | 0.15 | |
| A19 | 1 376 | α-古巴烯 α-Copaene | 7.35 | 19.05 | 22.94 | 2.48 | 12.66 | |
| A20 | 1 384 | β-波旁烯 β-Bourbonene | 1.40 | 2.11 | 3.32 | 0.41 | 2.48 | |
| A21 | 1 390 | β-荜澄茄油烯 β-Cubebene | 3.34 | 8.29 | 3.85 | 8.80 | 5.13 | |
| A22 | 1 393 | β-榄香烯β-Elemene | 6.84 | 2.79 | 2.10 | 6.40 | 4.02 | |
| A23 | 1 407 | 2,6-二甲基-6-(4-甲基- 3-戊烯基) 2,6-Dimethyl-6-(4-methyl-3-pentenyl)bicyclo[3.1.1]hept-2-ene | 0.65 | 0.52 | 0.24 | 0.29 | – | |
| A24 | 1 411 | (-)-α-古芸烯 (-)-α-Gurjunene | 0.35 | 1.64 | 1.09 | 0.64 | 1.31 | |
| A25 | 1 418 | (1S,5S)-4,6-二甲基-6- (4-甲基戊-3-烯基)双环 [3.1.1]庚-3-烯 (1S,5S)-4,6-Dimethyl-6-(4-methylpent-3-enyl)bicyclo[3.1.1]hept-3-ene | 0.65 | 0.29 | 0.35 | 3.28 | – | |
| A26 | 1 422 | β-石竹烯β-Caryophyllene | 8.01 | 3.33 | 5.58 | 10.42 | 5.70 | |
| A27 | 1 448 | 马兜铃烯Aristolene | 2.14 | 1.47 | 2.84 | 0.17 | 2.14 | |
| A28 | 1 454 | γ-广藿香烯γ-Patchoulene | 1.68 | 1.06 | 2.32 | – | 1.58 | |
| A29 | 1 459 | α-葎草烯α-Humulene | 1.87 | 2.91 | 2.48 | 24.41 | 3.90 | |
| A30 | 1 463 | β-倍半水芹烯 β-Sesquiphellandrene | 0.79 | 0.11 | – | – | – | |
| A31 | 1 466 | 香树烯(-)-Alloaromadendrene | 0.57 | 1.62 | 2.06 | – | 1.02 | |
| A32 | 1 475 | α-Himachalene | – | – | – | – | 0.53 | |
| A33 | 1 494 | (+)-β-瑟林烯 (+)-β-Selinene | 3.76 | 2.67 | 0.54 | 1.87 | 3.01 | |
| A34 | 1 489 | 右旋大根香叶烯 (+)- Germacrene D | 6.50 | 3.70 | 9.41 | 2.29 | 6.13 | |
| A35 | 1 498 | 愈创木烯Guaiacene | 3.09 | 1.58 | 0.14 | 0.70 | 1.19 | |
| A36 | 1 413 | (+)-γ-古芸烯 (+)-γ-Gurjunene | – | – | – | – | 3.71 | |
| A37 | 1 428 | (-)-β-花柏烯 (-)-β-Chamigrene | – | – | – | – | 2.50 | |
| A38 | 1 435 | γ-榄香烯γ-Elemene | – | – | – | – | 0.67 | |
| A39 | 1 505 | 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-cyclohexane | 5.09 | 7.03 | 6.42 | 0.42 | 6.43 | |
| A40 | 1 510 | (+)-香橙烯 (+)-Aromadendrene | 0.66 | 0.67 | 1.00 | 0.13 | – | |
| 类别 Class | 编号 No. | 保留指数 Retention index | 化合物 Compounds | 相对含量/% Relative content | ||||
| 浙江楠 P. chekian- gensis | 小叶桢楠 P. zhennan f. oblong | 大叶桢楠 P. zhennan f. elliptical | 紫楠 P. sheareri | 闽楠 P. bour- nei | ||||
| A41 | 1 490 | -Azulene,1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethylidene)-, (1S,4S)- | – | 0.13 | – | 0.70 | – | |
| A42 | 1 523 | (+)-γ-荜澄茄烯 (+)-γ-Cadinene | – | – | – | – | 0.53 | |
| A43 | 1 533 | δ-荜澄茄烯 δ-Cadinene | 6.01 | 1.87 | 3.57 | 1.12 | 1.81 | |
| A44 | 1 609 | α-广藿香烯 α-Patchoulene | 0.13 | 0.41 | 0.11 | 0.25 | 0.42 | |
| A45 | 1 621 | 环氧化蛇麻烯Ⅱ Humulene1,2-epoxide | 0.18 | 0.27 | 0.14 | 0.55 | – | |
| 醛类 Aldehydes | B1 | 1 645 | 2-甲基-4-戊醛 2-Methylpent-4-enal | 1.49 | 0.69 | 0.25 | 0.10 | 0.51 |
| B2 | 1 124 | 壬醛 Nonanal | 0.13 | – | – | – | 0.12 | |
| B3 | 1 210 | 癸醛 Decanal | 0.17 | 0.35 | 0.27 | – | – | |
| B4 | 1 305 | 10-十一烯醛10-Undecenal | – | – | – | – | 0.13 | |
| B5 | 1 310 | 十一醛 Undecanal | – | 0.33 | – | – | 0.13 | |
| B6 | 1 412 | 十二醛 Lauryl aldehyde | 0.16 | 1.02 | 0.13 | – | – | |
| B7 | 1 520 | 十四烷醛 Tetradecanal | – | – | – | 0.17 | – | |
| 醇类 Alcohols | C1 | 2 510 | 反式-3-己烯-1-醇 Trans-3-Hexen-1-ol | 2.08 | 1.57 | 1.02 | 1.51 | 1.50 |
| C2 | 2 738 | 3-甲基-4-戊醇 3-Methylpent-4-en-1-ol | 0.22 | 0.43 | – | 0.28 | – | |
| C3 | 1 409 | 环十二醇Cyclododecanol | – | – | – | – | 0.39 | |
| C4 | 2 714 | 叶醇cis-3-Hexen-1-ol | 0.17 | – | 0.36 | – | – | |
| 萜烯醇类 | D1 | 1 035 | 桉叶油醇 Cineole | 0.10 | – | – | 3.97 | – |
| Terpene alcohols | D2 | 1 108 | 芳樟醇 Linalool | 0.43 | – | – | – | – |
| D3 | 1 094 | 橙花醇 Nerol | – | – | – | – | 0.14 | |
| D4 | 1 295 | 紫苏醇 Perillyl alcohol | – | – | – | – | 0.13 | |
| D5 | 1 540 | α-檀香醇 α-Santalol | – | 0.13 | 0.11 | – | – | |
| D6 | 1 526 | α-人参烯 α-Panasinsanene | 0.11 | – | – | – | – | |
| D7 | 1 584 | 4(15),5,10(14)-大根香叶三 烯-1-醇 4(15),5,10(14)-Germacratrien-1-ol | – | 0.14 | 0.13 | – | – | |
| D8 | 1 590 | 桉油烯醇 Spathulenol | – | 0.68 | 0.22 | – | 0.47 | |
| D9 | 1 633 | Β-桉叶醇 β-Eudesmol | 0.16 | 0.10 | – | – | – | |
| D10 | 1 650 | 1aS,4aS,7R,7aS,7bS)-1,1,7- Trimethyl-4-methylidene-1a,2,3, 4a,5,6,7a,7b-octahydrocyclopropa [h]azulen-7-ol | – | 0.11 | – | – | – | |
| 酯类 Esters | E1 | 1 027 | (E)-3-己烯-1-醇乙酸酯 Trans-3-hexenyl acetate | 0.26 | 0.21 | 0.54 | 0.49 | 3.57 |
| E2 | 1 028 | 乙酸叶醇酯 cis-3-Hexenyl acetate | – | 0.10 | – | – | – | |
| 萜烯酯类 | F1 | 1 265 | 乙酸芳樟酯 Linalyl acetate | 0.23 | 0.41 | 0.17 | – | 0.91 |
| Terpene esters | F2 | 1 299 | 乙酸龙脑酯 Bornyl acetate | 3.70 | 0.98 | 0.62 | 5.10 | – |
| F3 | 1 292 | 左旋乙酸冰片酯 L-Bornyl acetate | – | – | – | – | 2.24 | |
| 萘类 Naphthalenes | G1 | 1 517 | (1aR)-1aβ,2,3,3a,4,5,6,7bβ- Octahydro-1,1,3aβ,7-tetramethyl- 1H-cyclopropa[a]naphthalene | 1.85 | 0.89 | 1.59 | 0.35 | – |
| G2 | 1 542 | 1,2,3,4,4a,7-Hexahydro-1,6- dimethyl-4-(1-methylethyl)- naphthalene | 0.35 | 0.30 | 0.29 | – | 0.20 | |
| G3 | 1 548 | 1,2,4a,5,6,8a-Hexahydro-4,7- dimethyl-1-(1-methylethyl) napthtalene | 0.15 | 0.14 | 0.24 | 0.11 | 0.41 | |
| G4 | 1 578 | (8aα)-Decahydro-1α-isopropyl- 3aα-methyl-7-methylene-4α,8α- epoxyazulene | – | 0.19 | 0.10 | – | 0.42 | |
| 类别 Class | 编号 No. | 保留指数 Retention index | 化合物 Compounds | 相对含量/% Relative content | ||||
| 浙江楠 P. chekian- gensis | 小叶桢楠 P. zhennan f. oblong | 大叶桢楠 P. zhennan f. elliptical | 紫楠 P. sheareri | 闽楠 P. bour- nei | ||||
| 烷烃类 Alkanes | H1 | 1 303 | 正十三烷Tridecane | – | – | – | 0.10 | 0.13 |
| H2 | 1 308 | 环己烷Cyclohexane | – | – | – | – | 0.67 | |
| H3 | 1 556 | (E)-2-(6,10-Timethylundeca-1,5,9- trien-2-yl)oxirane | 0.11 | – | – | – | – | |
| 有机酸 Organic acid | I1 | 1 283 | (1R,4S)-1,7,7-Trimethylbicyclo [2.2.1]heptan-2-yl acetate | – | – | – | – | 0.33 |
| 氧化物 Oxide | J1 | 1 595 | 石竹素Caryophyllene oxide | 0.49 | 0.34 | 0.22 | 0.21 | 0.36 |
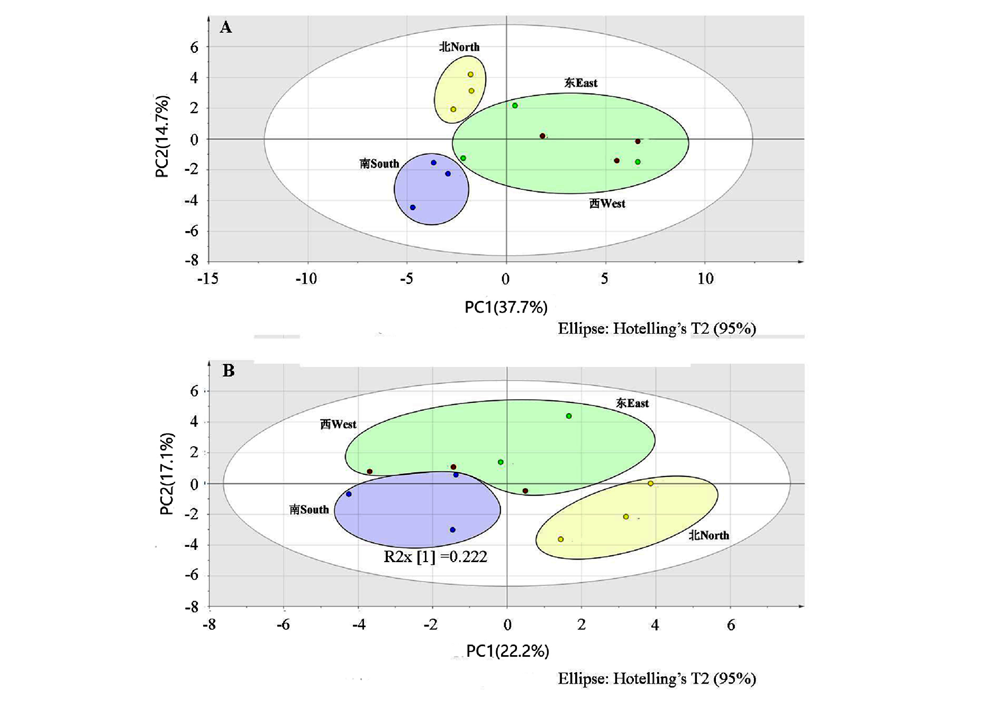
图2 夏季(A)和秋季(B)浙江楠不同方向叶片挥发性成分的OPLS-DA分析
Fig. 2 OPLS-DA analysis of volatile components in leaves Phoebe chekiangensis in different directions in summer(A) and autumn(B)
| 化合物 Compounds | 相对含量/ % Relative content | 化合物 Compounds | 相对含量/% Relative content | ||
|---|---|---|---|---|---|
| 夏季 Summer | 秋季 Autumn | 夏季 Summer | 秋季 Autumn | ||
| 莰烯Camphene | 4.85 ± 2.09** | 1.03 ± 0.13 | α-葎草烯 α-Humulene | 1.61 ± 0.18 | 1.42 ± 0.04 |
| 甘香烯Caryophyllene | 5.50 ± 1.67 | 3.20 ± 1.87 | (-)-α-荜澄茄油烯 (-)-α-Cubebene | 2.46 ± 0.03 | 1.76 ± 0.18 |
| α-古巴烯α-Copaene | 7.49 ± 2.09* | 6.30 ± 1.46 | 右旋大根香叶烯 (+)-Germacrene D | 5.57 ± 2.23 | 5.52 ± 2.58 |
| β-榄香烯β-Elemene | 8.81 ± 2.97 | 8.91 ± 1.53 | (-)-α-古芸烯 (-)-α-Gurjunene | 4.10 ± 2.17* | 2.49 ± 1.62 |
| β-石竹烯β-Caryophyllene | 11.87 ± 2.31* | 8.92 ± 1.68 | δ-荜澄茄烯 δ-Cadinene | 6.44 ± 2.97** | 3.22 ± 1.93 |
| 马兜铃烯Aristolene | 2.44 ± 0.18 | 2.32 ± 0.32 | 芹子烯 Apigenine | 2.26 ± 2.05 | 2.87 ± 2.55 |
| α-蒎烯α-Pinene | 0.49 ± 0.05** | 4.40 ± 0.63 | 佛术烯 Eremophilene | 1.69 ± 0.06 | 1.79 ± 0.40 |
表2 夏季和秋季浙江楠叶片的主要挥发性成分比较
Table 2 Comparison of main volatile components in leaves of Phoebe chekiangensis between summer and autumn
| 化合物 Compounds | 相对含量/ % Relative content | 化合物 Compounds | 相对含量/% Relative content | ||
|---|---|---|---|---|---|
| 夏季 Summer | 秋季 Autumn | 夏季 Summer | 秋季 Autumn | ||
| 莰烯Camphene | 4.85 ± 2.09** | 1.03 ± 0.13 | α-葎草烯 α-Humulene | 1.61 ± 0.18 | 1.42 ± 0.04 |
| 甘香烯Caryophyllene | 5.50 ± 1.67 | 3.20 ± 1.87 | (-)-α-荜澄茄油烯 (-)-α-Cubebene | 2.46 ± 0.03 | 1.76 ± 0.18 |
| α-古巴烯α-Copaene | 7.49 ± 2.09* | 6.30 ± 1.46 | 右旋大根香叶烯 (+)-Germacrene D | 5.57 ± 2.23 | 5.52 ± 2.58 |
| β-榄香烯β-Elemene | 8.81 ± 2.97 | 8.91 ± 1.53 | (-)-α-古芸烯 (-)-α-Gurjunene | 4.10 ± 2.17* | 2.49 ± 1.62 |
| β-石竹烯β-Caryophyllene | 11.87 ± 2.31* | 8.92 ± 1.68 | δ-荜澄茄烯 δ-Cadinene | 6.44 ± 2.97** | 3.22 ± 1.93 |
| 马兜铃烯Aristolene | 2.44 ± 0.18 | 2.32 ± 0.32 | 芹子烯 Apigenine | 2.26 ± 2.05 | 2.87 ± 2.55 |
| α-蒎烯α-Pinene | 0.49 ± 0.05** | 4.40 ± 0.63 | 佛术烯 Eremophilene | 1.69 ± 0.06 | 1.79 ± 0.40 |
| 化合物 Compounds | 相对含量/% Relative content | |||
|---|---|---|---|---|
| 15 ℃ | 25 ℃ | 35 ℃ | 45 ℃ | |
| 蒎烯 Pinene | 7.19 ± 1.43 a | 5.86 ± 1.83 a | 6.38 ± 1.89 a | 6.30 ± 1.41 a |
| α-古巴烯 α-Copaene | 4.22 ± 0.19 a | 3.34 ± 0.73 a | 2.70 ± 0.19 ab | 2.36 ± 0.01 b |
| β-蒎烯 β-Pinene | 3.66 ± 0.96 a | 3.22 ± 0.13 a | 3.00 ± 0.76 a | 2.79 ± 1.10 a |
| (-)-异喇叭烯 (-)-isoledene | 1.10 ± 0.11 a | 1.15 ± 0.04 a | 1.17 ± 0.11 a | 1.20 ± 0.05 a |
| (-)-α-荜澄茄油烯 (-)-α-Cubebene | 3.41 ± 0.29 a | 2.98 ± 0.47 ab | 2.44 ± 1.21 ab | 2.08 ± 0.11 b |
| β-榄香烯 β-Elemene | 5.19 ± 1.27 a | 5.29 ± 0.94 a | 5.46 ± 1.16 a | 6.90 ± 1.04 a |
| β-石竹烯 β-Caryophyllene | 18.82 ± 0.83 b | 22.97 ± 0.18 a | 20.66 ± 1.85 b | 19.95 ± 1.98 b |
| (1S,4aR,8aR)-7-Isopropylidene-1,4a-dimethyldecahydro-1-naphthalen ol | 1.44 ± 1.09 a | 1.97 ± 0.32 a | 1.60 ± 0.38 a | 1.94 ± 0.52 a |
| 马兜铃烯 Aristolene | 1.13 ± 0.11 a | 1.35 ± 0.16 a | 1.13 ± 0.11 a | 0.89 ± 0.13 a |
| α-葎草烯 α-Humulene | 16.40 ± 0.29 b | 17.98 ± 0.48 ab | 19.83 ± 0.36 a | 18.91 ± 1.53 a |
| (+)-β-瑟林烯 (+)-β-Selinene | 3.41 ± 0.12 a | 3.41 ± 0.79 a | 2.59 ± 0.45 b | 3.39 ± 0.19 a |
| α-瑟林烯 α-Selinene | 3.90 ± 0.14 b | 4.29 ± 0.11 b | 4.77 ± 0.35 b | 6.61 ± 0.49 a |
| (+)-γ-古芸烯 (+)-γ-Gurjunene | 5.04 ± 0.08 a | 5.14 ± 1.18 a | 3.22 ± 0.17 b | 4.48 ± 0.17 ab |
| 乙酸龙脑酯 Bornyl acetate | 1.04 ± 0.04 a | 0.97 ± 0.02 a | 1.01 ± 0.01 a | 0.87 ± 0.02 a |
| α-法呢烯 α- Farnesene | 2.13 ± 0.56 a | 1.30 ± 0.57 b | 1.13 ± 0.12 b | 0.71 ± 0.12 b |
| 1-ethenyl-1-methyl-4-propan-2-ylidene-2-prop-1-en-2-ylcyclohexane | 3.15 ± 1.82 a | 2.51 ± 0.51 a | 3.46 ± 0.37 a | 2.24 ± 0.46 a |
表3 不同栽培温度下浙江楠叶片挥发性化合物的相对含量
Table 3 The relative contents of volatile compounds in leaves of Phoebe chekiangensis under different temperatures
| 化合物 Compounds | 相对含量/% Relative content | |||
|---|---|---|---|---|
| 15 ℃ | 25 ℃ | 35 ℃ | 45 ℃ | |
| 蒎烯 Pinene | 7.19 ± 1.43 a | 5.86 ± 1.83 a | 6.38 ± 1.89 a | 6.30 ± 1.41 a |
| α-古巴烯 α-Copaene | 4.22 ± 0.19 a | 3.34 ± 0.73 a | 2.70 ± 0.19 ab | 2.36 ± 0.01 b |
| β-蒎烯 β-Pinene | 3.66 ± 0.96 a | 3.22 ± 0.13 a | 3.00 ± 0.76 a | 2.79 ± 1.10 a |
| (-)-异喇叭烯 (-)-isoledene | 1.10 ± 0.11 a | 1.15 ± 0.04 a | 1.17 ± 0.11 a | 1.20 ± 0.05 a |
| (-)-α-荜澄茄油烯 (-)-α-Cubebene | 3.41 ± 0.29 a | 2.98 ± 0.47 ab | 2.44 ± 1.21 ab | 2.08 ± 0.11 b |
| β-榄香烯 β-Elemene | 5.19 ± 1.27 a | 5.29 ± 0.94 a | 5.46 ± 1.16 a | 6.90 ± 1.04 a |
| β-石竹烯 β-Caryophyllene | 18.82 ± 0.83 b | 22.97 ± 0.18 a | 20.66 ± 1.85 b | 19.95 ± 1.98 b |
| (1S,4aR,8aR)-7-Isopropylidene-1,4a-dimethyldecahydro-1-naphthalen ol | 1.44 ± 1.09 a | 1.97 ± 0.32 a | 1.60 ± 0.38 a | 1.94 ± 0.52 a |
| 马兜铃烯 Aristolene | 1.13 ± 0.11 a | 1.35 ± 0.16 a | 1.13 ± 0.11 a | 0.89 ± 0.13 a |
| α-葎草烯 α-Humulene | 16.40 ± 0.29 b | 17.98 ± 0.48 ab | 19.83 ± 0.36 a | 18.91 ± 1.53 a |
| (+)-β-瑟林烯 (+)-β-Selinene | 3.41 ± 0.12 a | 3.41 ± 0.79 a | 2.59 ± 0.45 b | 3.39 ± 0.19 a |
| α-瑟林烯 α-Selinene | 3.90 ± 0.14 b | 4.29 ± 0.11 b | 4.77 ± 0.35 b | 6.61 ± 0.49 a |
| (+)-γ-古芸烯 (+)-γ-Gurjunene | 5.04 ± 0.08 a | 5.14 ± 1.18 a | 3.22 ± 0.17 b | 4.48 ± 0.17 ab |
| 乙酸龙脑酯 Bornyl acetate | 1.04 ± 0.04 a | 0.97 ± 0.02 a | 1.01 ± 0.01 a | 0.87 ± 0.02 a |
| α-法呢烯 α- Farnesene | 2.13 ± 0.56 a | 1.30 ± 0.57 b | 1.13 ± 0.12 b | 0.71 ± 0.12 b |
| 1-ethenyl-1-methyl-4-propan-2-ylidene-2-prop-1-en-2-ylcyclohexane | 3.15 ± 1.82 a | 2.51 ± 0.51 a | 3.46 ± 0.37 a | 2.24 ± 0.46 a |
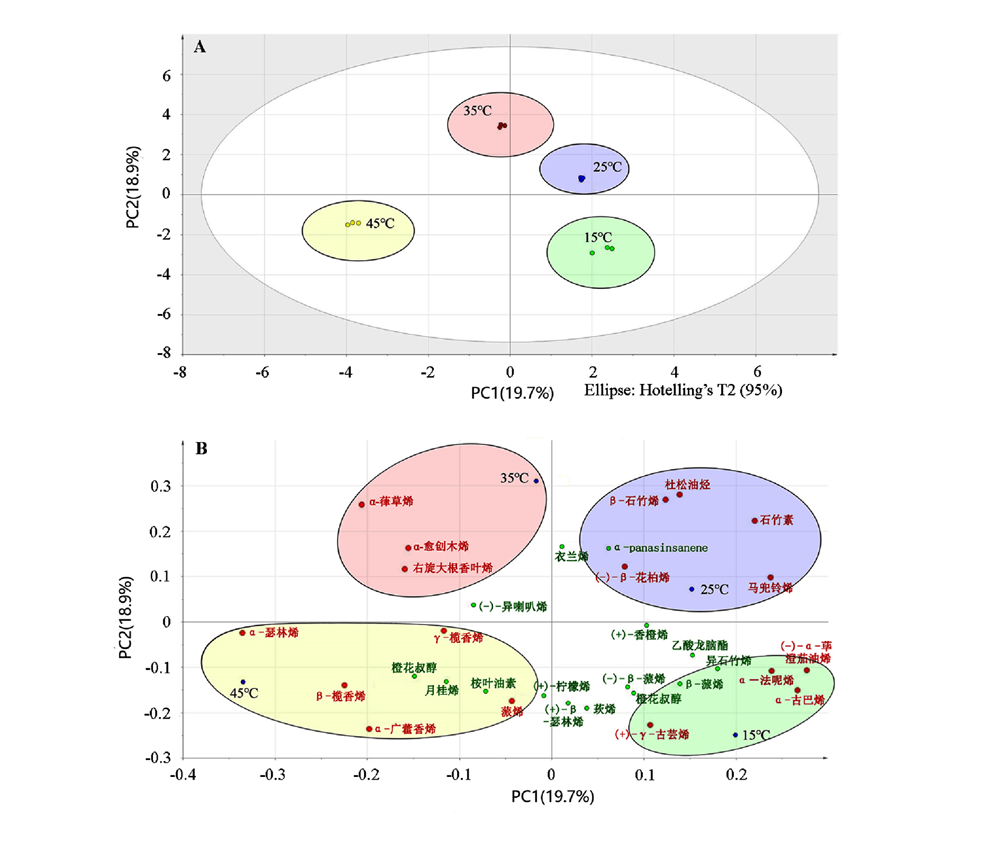
图3 浙江楠叶片不同温度下的挥发性成分OPLS-DA(A)及特征成分分析(B)
Fig. 3 OPLS-DA(A)and characteristic component analysis(B)of volatile components in leaves of Phoebe chekiangensis under different temperatures
| 化合物 Compounds | 相对含量/% Relative contents/% | |||||
|---|---|---|---|---|---|---|
| 8:00 | 10:00 | 12:00 | 14:00 | 16:00 | 18:00 | |
| 右旋大根香叶烯(+)-Germacrene D | 9.79 | 13.00 | 7.72 | 8.57 | 15.64 | 10.39 |
| β-石竹烯 β-Caryophyllene | 12.53 | 12.85 | 10.56 | 13.92 | 13.08 | 13.38 |
| α-古巴烯 α-Copaene | 5.87 | 4.95 | 4.67 | 7.98 | 4.05 | 6.49 |
| β-荜澄茄烯 β-Cadinene | 0.92 | 1.00 | 0.52 | 0.89 | 0.96 | 1.02 |
| γ-摩勒烯 γ-muurolene | 2.19 | 2.45 | 4.72 | 3.00 | 2.28 | 2.38 |
| β-榄香烯 β-Elemene | 6.95 | 8.40 | 5.87 | 8.58 | 8.44 | 7.74 |
| 叶醇cis-3-Hexen-1-ol | — | — | — | 3.07 | — | — |
| 桉油烯醇 Spathulenol | 0.17 | 0.10 | 0.19 | 0.13 | — | — |
| α-葎草烯 α-Humulene | 1.65 | 1.95 | 2.02 | 1.71 | 1.99 | 1.82 |
表4 浙江楠叶片挥发性成分的日变化
Table 4 Diurnal variation of volatile components in leaves of Phoebe chekiangensis
| 化合物 Compounds | 相对含量/% Relative contents/% | |||||
|---|---|---|---|---|---|---|
| 8:00 | 10:00 | 12:00 | 14:00 | 16:00 | 18:00 | |
| 右旋大根香叶烯(+)-Germacrene D | 9.79 | 13.00 | 7.72 | 8.57 | 15.64 | 10.39 |
| β-石竹烯 β-Caryophyllene | 12.53 | 12.85 | 10.56 | 13.92 | 13.08 | 13.38 |
| α-古巴烯 α-Copaene | 5.87 | 4.95 | 4.67 | 7.98 | 4.05 | 6.49 |
| β-荜澄茄烯 β-Cadinene | 0.92 | 1.00 | 0.52 | 0.89 | 0.96 | 1.02 |
| γ-摩勒烯 γ-muurolene | 2.19 | 2.45 | 4.72 | 3.00 | 2.28 | 2.38 |
| β-榄香烯 β-Elemene | 6.95 | 8.40 | 5.87 | 8.58 | 8.44 | 7.74 |
| 叶醇cis-3-Hexen-1-ol | — | — | — | 3.07 | — | — |
| 桉油烯醇 Spathulenol | 0.17 | 0.10 | 0.19 | 0.13 | — | — |
| α-葎草烯 α-Humulene | 1.65 | 1.95 | 2.02 | 1.71 | 1.99 | 1.82 |
| [1] |
Bai M, Chen J J, Xu W, Dong S H, Song S J. 2020. Elephantopinolide A-P,germacrane-type sesquiterpene lactones from Elephantopus scaber induce apoptosis,autophagy and G2/M phase arrest in hepatocellular carcinoma cells. European Journal of Medicinal Chemistry, 198:112362.
doi: 10.1016/j.ejmech.2020.112362 URL |
| [2] | Bian Jing-jun, Cheng Mi-mi, Luo Si-yuan, Chen Si-ling, Liu Shi-yao, Bai Zhi-chuan. 2014. GC/MS analysis of volatile substances in essential oil of Lindera megaphylla blade and its application. Journal of Southwest University(Natural Science Edition), 36 (10):82-88. (in Chinese) |
| 卞京军, 程密密, 罗思源, 陈思伶, 刘世尧, 白志川. 2014. 黑壳楠叶片精油挥发性成分的GC/MS鉴定与应用分析. 西南大学学报(自然科学版), 36 (10):82-88. | |
| [3] | Cao Ai-ling, Zhou Jian, Zhang Yong, Cao Zhe, Shu Cheng-rong. 2019. Effects of β-elemene combined with docetaxel on the proliferation and apoptosis of human cervical cancer HeLa cells. Oncology Pharmacy, 9 (3):401-406. (in Chinese) |
| 曹爱玲, 周剑, 章永, 曹喆, 舒诚荣. 2019. β-榄香烯联合多西紫杉醇对人宫颈癌HeLa细胞增殖和凋亡的影响. 肿瘤药学, 9 (3):401-406. | |
| [4] |
Cecchi T, Alfei B. 2013. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME-GC-MS:newly identified compounds,flavors molecular markers,and terpenic profile. Food Chemistry, 141 (3):2025-2035.
doi: 10.1016/j.foodchem.2013.05.090 URL |
| [5] | Cen Bo, Li Long-sheng, Duan Wen-gui, Lin Gui-shan, Chen Ming, Lu Shun-zhong. 2020. Synthesis and antifungal activities of novel α-pinene-based benzene sulfonamide compounds. Synthetic Chemistry, 28 (3):174-180. (in Chinese) |
| 岑波, 李龙生, 段文贵, 林桂汕, 陈铭, 陆顺忠. 2020. 新型α-蒎烯基苯磺酰胺类化合物的合成及其抗真菌活性. 合成化学, 28 (3):174-180. | |
| [6] |
Chang X, Alderson P G, Wright C J. 2005. Effect of temperature integration on the growth and volatile oil content of basil(Ocimum basilicum L). Journal of Horticultural Science and Biotechnology, 80 (5):593-598.
doi: 10.1080/14620316.2005.11511983 URL |
| [7] | Cui Ting-ting, Shan Chang-song, Wu Peng, Zhou Tao. 2015. The analysis of volatile flavor compounds of honeysuckle and red honeysuckle. Acta Horticulturae Sinica, 42 (11):2283-2290. (in Chinese) |
| 崔婷婷, 单长松, 吴澎, 周涛. 2015. 金银花和红银花挥发性成分的顶空固相微萃取气质联用检测与比较. 园艺学报, 42 (11):2283-2290. | |
| [8] | Deng X H, Xie P F, Peng X H, Yi J H, Zhou J H, Zhou Q M, Pu W X, Dai Y G. 2010. Effects of soil,climate,and their interaction on some neutral volatile aroma components in flue-cured tobacco leaves from high quality tobacco planting regions of Hunan Province. The Journal of Applied Ecology, 21 (8):2063-2071. |
| [9] | Ding Q, Bao J, Zhao W, Hu Y, Lu J, Chen X. 2015. Natural autophagy regulators in cancer therapy:a review. Phytochemistry Reviews, 14 (1):137-154. |
| [10] |
Ding W, Liping N, Xing H, Wei Z, Zhoua Q, Nong R, Chen J. 2019. Essential oil extracted from leaf of Phoebe bournei(Hemsl.)Yang:chemical constituents,antitumor,antibacterial,hypoglycemic activities. Natural Product Research, 34 (17):2524-2527.
doi: 10.1080/14786419.2018.1542393 URL |
| [11] | Ding Wen, Ning Li-ping, Yang Wei, Xiong Yan, Zhang Shuai, Liu Jiang. 2017. Study on chemical compositions of phytoncidere and essential oil and bioactivity of essential oil from Phoebe zhennan. Journal of Northwest A & F University(Natural Science Edition), 45 (9):123-128. (in Chinese) |
| 丁文, 宁莉萍, 杨威, 熊燕, 张帅, 刘江. 2017. 桢楠精油、精气化学成分及精油生物活性研究. 西北农林科技大学学报(自然科学版), 45 (9):123-128. | |
| [12] |
Han Jun-li, Li Zhen-qiu, Liu Ben-ye, Wang Hong, Li Guo-feng, Ye He-chun. 2007. Metabolic engineering of terpenoids in plants. Chinese Journal of Biotechnology, 23 (4):561-569. (in Chinese)
pmid: 17822023 |
|
韩军丽, 李振秋, 刘本叶, 王红, 李国凤, 叶和春. 2007. 植物萜类代谢工程. 生物工程学报, 23 (4):561-569.
pmid: 17822023 |
|
| [13] | Heng L, De F W, Yu J C, Min S Y. 2020. β-Caryophyllene inhibits high glucose-induced oxidative stress,inflammation and extracellular matrix accumulation in mesangial cells. International Immunopharmacology, 84:106-556. |
| [14] | He Dong, Wang Min. 2019. Research progress on extraction and analysis methods of plant volatile components. Modern Food,(1):28-31. (in Chinese) |
| 何东, 王敏. 2019. 植物挥发性成分提取及分析方法研究进展. 现代食品,(1):28-31. | |
| [15] |
Kyoung S C, Young R L, Kyungho L, Jaeseok L, Jang H L, Im-Soon L. 2017. Terpenes from forests and human health. Toxicological Research, 33 (2):97-106.
doi: 10.5487/TR.2017.33.2.097 pmid: 28443180 |
| [16] |
Köksal M, Jin Y, Coates R M, Croteau R, Christianson D W. 2011. Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis. Nature, 469 (7328):116-120.
doi: 10.1038/nature09628 URL |
| [17] | Jin Ya-nan, Du Xi-wei, Yang Rui-xia. 2019. Inhibition effects of β-elemene injection combined with apatinib mesylate on growth of breast cancer cells. Journal of Clinical and Experimental Medicine,(20):2146-2150. (in Chinese) |
| 晋亚楠, 杜喜维, 杨瑞霞. 2019. β-榄香烯注射液联合甲磺酸阿帕替尼对乳腺癌细胞生长抑制作用的研究. 临床和实验医学杂志,(20):2146-2150. | |
| [18] | Li Dong-lin, Jin Ya-qin, Xiang Qi-bai. 2004. The geographical distribution,research status and developmental utilization prospect of Phoebe Nees plant resource of our country. Journal of Fujian Forestry and Technology, 31 (1):5-9. (in Chinese) |
| 李冬林, 金雅琴, 向其柏. 2004. 我国楠木属植物资源的地理分布、研究现状和开发利用前景. 福建林业科技, 31 (1):5-9. | |
| [19] | Li Yang, Jiang Guang-yu, Wang Hai-yang. 2014. On analysis of bactericidal ability and effective component of four garden trees. Journal of Southwest China Normal University(Natural Science Edition), 39 (6):29-34. (in Chinese) |
| 李阳, 江广渝, 王海洋. 2014. 4种樟科园林树种挥发性物质杀菌能力测定及有效成分分析. 西南师范大学学报(自然科学版), 39 (6):29-34. | |
| [20] | Liu Wen-han, He Jing-jing, Teng Yuan-jie. 2013. Analysis of volatile components of Atractylodes macrocephala Koidz by Headspace Liquid Phase-Liquid-Phase extraction coupled with gas chromatography-mass spectrometry. Chinese Journal of Analytical Chemistry, 41 (8):1226-1231. (in Chinese) |
| 刘文涵, 何晶晶, 滕渊洁. 2013. 顶空液液萃取–气相色谱–质谱法用于白术挥发性成分的分析. 分析化学, 41 (8):1226-1231. | |
| [21] | Lu An-xia, Zhou Xin-ru, Ye Yu-long, Li Xiao-lian, Xie Guan-hua, Wang Bei, Tong Hua-rong. 2020. Changes of sensory characteristic and volatiles of harvested flowers of Chimonanthus praecox during spreading process. Acta Horticulturae Sinica, 47 (1):73-84. (in Chinese) |
| 陆安霞, 周心如, 叶玉龙, 李小恋, 谢关华, 汪蓓, 童华荣. 2020. 蜡梅花离体摊放过程中香气感官评价和挥发性物质分析. 园艺学报, 47 (1):73-84. | |
| [22] | Luo Si-yuan. 2015. GC-MS Analysis of the components of volatile oils from the fresh leaves of Beilschmiedia yaanica. Journal of Southwest University, 37 (3):166-172. (in Chinese) |
| 罗思源. 2015. 雅安琼楠鲜叶挥发油成分的GC-MS分析. 西南大学学报, 37 (3):166-172. | |
| [23] | Mo Li-ping, Li Tian-yu, Zhang Chao-han. 2019. Effect of β-elemene on chemotherapy sensitization and apoptosis of colorectal cancer LoVo cell line. Chinese Journal of Experimental Surgery, 36 (6):1117. (in Chinese) |
| 莫丽萍, 李天煜, 袁朝汉. 2019. β-榄香烯对结直肠癌LoVo细胞株化疗增敏及细胞凋亡作用的研究. 中华实验外科杂志, 36 (6):1117. | |
| [24] |
Ouyang Ting, Mai Xi. 2010. Analysis of the chemical constituents of essential oil from Chimonanthus zhejiangensis by GC-MS. Journal of Chinese Medicinal Materials, 33 (3):385-387. (in Chinese)
pmid: 20681305 |
|
欧阳婷, 麦曦. 2010. 浙江蜡梅叶挥发油化学成分GC-MS分析. 中药材, 33 (3):385-387.
pmid: 20681305 |
|
| [25] | Qin Wei. 2015. The effect of ecological environment on effective components of plants. Agriculture and Technology, 35 (16):23. (in Chinese) |
| 秦维. 2015. 生态环境对植物有效成分的影响. 农业与技术, 35 (16):23. | |
| [26] | Xia Guo-hua, Mei Ai-jun. 2018. Illustrated rare wild plants in Lin’an. Beijing: China Forestry Publishing House:60-64. (in Chinese) |
| 夏国华, 梅爱君. 2018. 临安珍稀野生植物图鉴. 北京: 中国林业出版社:60-64. | |
| [27] | Xia Zhong-di, Yu Jun-long. 2000. Effect of α-Pinene on candida albicans biosynthesis. China Journal of Modern Medicine, 10 (1):48-50. (in Chinese) |
| 夏忠弟, 余俊龙. 2000. α-蒎烯对白色念珠菌生物合成的影响. 中国现代医学杂志, 10 (1):48-50. | |
| [28] | Xu Meng, Zhang Jing-wei, Wu Ling-shang, Liu Jing-jing, Si Jin-ping, Zhang Xin-feng. 2016. Determination of volatile components from Chimonanthus flowers by HS-SPME-GC-MS. Scientia Silvae Sinicae, 52 (12):59-65. (in Chinese) |
| 徐萌, 张经纬, 吴令上, 刘京晶, 斯金平, 张新凤. 2016. HS-SPME-GC-MS联用测定蜡梅属植物花的挥发性成分. 林业科学, 52 (12):59-65. | |
| [29] | Yao Chen-yang, Ge Hong, Wu Hua, Jia Rui-dong, Zhao Xin, Lü Ying-min, Yang Shu-hua. 2019. Petal volatile components among different varieties of Rosa rugosa. Acta Horticulturae Sinica, 46 (2):375-384. (in Chinese) |
| 姚晨阳, 葛红, 吴华, 贾瑞冬, 赵鑫, 吕英民, 杨树华. 2019. 玫瑰不同品种花瓣挥发性成分分析. 园艺学报, 46 (2):375-384. | |
| [30] | Ye Bang-min, Pan Ri-gui, Ye Bang-zhi. 2010. Lauraceae plant resources in Qingyuan County and their application in gardens. Modern Agricultural Science and Technology,(5):79-81. (in Chinese) |
| 叶帮民, 潘日贵, 叶邦志. 2010. 庆元县樟科植物资源及其在园林中的应用. 现代农业科技,(5):79-81. | |
| [31] | Zhu Yue-lin, Wang Wen-guang, Xiong Chang-jian. 2009. Comparsion on composition of different Rosa essential oil. Journal of Beijing University of Technology, 35 (9):1253-1257. (in Chinese) |
| 朱岳麟, 王文广, 熊常健. 2009. 玫瑰香精油化学成分分析. 北京工业大学学报, 35 (9):1253-1257. |
| [1] | 强文彦, 孟庆然, 张志国, 高文杰. 萱草不同品种花瓣挥发性物质的HS-SPME-GC-MS分析[J]. 园艺学报, 2023, 50(1): 116-130. |
| [2] | 李晓明, 于俊池, 王春夏. 露地、温室、温室遮阳下紫花和白花香青兰生长及次生代谢物比较[J]. 园艺学报, 2022, 49(6): 1363-1370. |
| [3] | 沈植国, 孙 萌, 袁德义, 程建明, 丁 鑫, 尚忠海, . 蜡梅科6种植物嫩梢挥发性成分的HS–SPME–GC–MS分析[J]. 园艺学报, 2020, 47(12): 2349-2361. |
| [4] | 姚晨阳1,2,葛 红2,吴 华3,贾瑞冬2,赵 鑫2,吕英民1,*,杨树华2,*. 玫瑰不同品种花瓣挥发性成分分析[J]. 园艺学报, 2019, 46(2): 375-384. |
| [5] | 李晓颍1,武军凯1,王海静1,张红霞2,郭学民2,*. ‘飞黄’玉兰花发育期各轮花被片挥发性成分分析[J]. 园艺学报, 2019, 46(10): 2009-2020. |
| [6] | 董 静,王桂霞,钟传飞,常琳琳,孙 健,张宏力,孙 瑞,石 琨,隗永青,张运涛*. 森林草莓醇酰基转移酶基因FvAATW2功能研究[J]. 园艺学报, 2018, 45(1): 41-50. |
| [7] | 陈君梅1,宋军阳1,*,何 洁2,顾秀容2,张 显2,*. 秦岭地区春兰和蕙兰的花挥发性成分研究[J]. 园艺学报, 2016, 43(12): 2461-2472. |
| [8] | 崔婷婷1,单长松1,吴 澎1,*,周 涛2,*. 金银花和红银花挥发性成分的顶空固相微萃取#br# 气质联用检测与比较[J]. 园艺学报, 2015, 42(11): 2283-2290. |
| [9] | 李国鹏;贾惠娟;王 强;张茂君;滕元文;. ‘小香水’梨果实后熟过程中挥发性组分分析[J]. 园艺学报, 2012, 39(1): 151-158. |
| [10] | 柴倩倩;王利军;吴本宏;范培格;段 伟;李绍华;. 中国李和樱桃李及其种间杂种果实香气成分分析[J]. 园艺学报, 2011, 38(12): 2357-2364. |
| [11] | 陈计峦;周珊;闫师杰;马永昆;胡小松. 丰水梨、砀山梨、南果梨的香气成分分析[J]. 园艺学报, 2005, 32(02): 301-303. |
| [12] | 范燕萍 余让才 黄 蕴 陈玉芬. 姜花挥发性成分的固相微萃取一气相色谱质谱分析[J]. 园艺学报, 2003, 30(4): 475-475. |
| [13] | 张 继;马君义;黄爱仑;姚 健;杨永利. 千里香杜鹃挥发性成分的分析研究[J]. 园艺学报, 2002, 29(4): 386-388. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司