
园艺学报 ›› 2021, Vol. 48 ›› Issue (3): 566-576.doi: 10.16420/j.issn.0513-353x.2020-0483
收稿日期:2020-09-04
出版日期:2021-03-25
发布日期:2021-04-02
通讯作者:
王秀云,夏宜平
E-mail:wxy.550@163.com;ypxia@zju.edu.cn
基金资助:
LI Zheng, LIU Bing, ZHOU Hong, WANG Xiuyun( ), XIA Yiping(
), XIA Yiping( )
)
Received:2020-09-04
Online:2021-03-25
Published:2021-04-02
Contact:
WANG Xiuyun,XIA Yiping
E-mail:wxy.550@163.com;ypxia@zju.edu.cn
摘要:
为了阐述RhRCA1基因的调控机制,以海南杜鹃(Rhododendron hainanense)3年生扦插苗为材料,分析RhRCA1高温响应和组织表达特性;并克隆获得RhRCA1的启动子序列,将该启动子分别驱动荧光素酶报告基因在烟草中瞬时表达、GUS报告基因在拟南芥中稳定表达,分析该启动子活性、组织特异性和热诱导性。结果显示:(1)25 ℃条件下,RhRCA1表达量极低,37 ℃处理后其表达量显著升高,呈现典型的热诱导特性,并且RhRCA1在绿色组织中的表达量显著高于非绿色组织,呈现组织特异性;(2)获得的启动子长1 624 bp,其含有多个非生物胁迫响应、光响应、组织特异性等相关元件;(3)构建RhRCA1启动子和萤光素酶融合的植物表达载体,在烟草叶片中瞬时表达,荧光成像结果表明RhRCA1启动子能强烈响应高温胁迫;(4)构建RhRCA1启动子和GUS融合的植物表达载体,转化拟南芥植株筛选至T3代,GUS染色结果显示,高温能显著诱导RhRCA1启动子在拟南芥子叶、成熟叶、茎、萼片、果荚等绿色组织中的表达。研究结果表明RhRCA1启动子是1个兼具高温诱导型和组织特异性的启动子,可应用于植物抗逆基因工程,提高植物在高温胁迫下的抗性。
中图分类号:
李铮, 刘冰, 周泓, 王秀云, 夏宜平. 海南杜鹃热诱导基因RhRCA1启动子的克隆与功能分析[J]. 园艺学报, 2021, 48(3): 566-576.
LI Zheng, LIU Bing, ZHOU Hong, WANG Xiuyun, XIA Yiping. Isolation and Function Analysis of the Promoter of a Thermal Inducible Gene RCA1 in Rhododendron hainanense[J]. Acta Horticulturae Sinica, 2021, 48(3): 566-576.
| 名称 Name | 序列 Sequence(5′-3′) | ||||
|---|---|---|---|---|---|
| prRCA1-F prRCA1-R | ACTCGGCACAGCTACTACC CAAAGGTGGAAACGGCAG | ||||
| RCA1-F RCA1-R 18S-F 18S-R | TGCTGGTTCAAGAGCAGGAG GTTGAGCTGCTTTGCCATAGA CGCATTCCCCACTGTATTAGAC CGTAACAAGGTTTCCGTAGGTG | ||||
| prRCA1-F-Hind Ⅲ prRCA1-R-Nco I prRCA1-LUC-F prRCA1-LUC-R | ACCTGCAGGCATGCAAGCTTCTACTACCAAGCACCTCCGC TTACCCTCAGATCTACCATGGAGAAATCAAGGGTCTGTTTGGGA GCTTGATATCGAATTCCTGCAGCTACTACCAAGCACCTCCGC GGATCCCCCGGGCTGCAGAGAAATCAAGGGTCTGTTTGGGA | ||||
| P3301-F-Hind Ⅲ P3301-R-Nco Ⅰ pGreen-LUC-F pGreen-LUC-R | ACCTGCAGGCATGCAAGCTT TTACCCTCAGATCTACCATGG GGCGATTAAGTTGGGTAACGC GGTTCCATCTTCCAGCGGATA | ||||
| P3301-F-Hind Ⅲ GUS-R | ACCTGCAGGCATGCAAGCTT CACGGGTTGGGGTTTCTACA |
表1 试验中使用的引物
Table 1 Primers used in this study
| 名称 Name | 序列 Sequence(5′-3′) | ||||
|---|---|---|---|---|---|
| prRCA1-F prRCA1-R | ACTCGGCACAGCTACTACC CAAAGGTGGAAACGGCAG | ||||
| RCA1-F RCA1-R 18S-F 18S-R | TGCTGGTTCAAGAGCAGGAG GTTGAGCTGCTTTGCCATAGA CGCATTCCCCACTGTATTAGAC CGTAACAAGGTTTCCGTAGGTG | ||||
| prRCA1-F-Hind Ⅲ prRCA1-R-Nco I prRCA1-LUC-F prRCA1-LUC-R | ACCTGCAGGCATGCAAGCTTCTACTACCAAGCACCTCCGC TTACCCTCAGATCTACCATGGAGAAATCAAGGGTCTGTTTGGGA GCTTGATATCGAATTCCTGCAGCTACTACCAAGCACCTCCGC GGATCCCCCGGGCTGCAGAGAAATCAAGGGTCTGTTTGGGA | ||||
| P3301-F-Hind Ⅲ P3301-R-Nco Ⅰ pGreen-LUC-F pGreen-LUC-R | ACCTGCAGGCATGCAAGCTT TTACCCTCAGATCTACCATGG GGCGATTAAGTTGGGTAACGC GGTTCCATCTTCCAGCGGATA | ||||
| P3301-F-Hind Ⅲ GUS-R | ACCTGCAGGCATGCAAGCTT CACGGGTTGGGGTTTCTACA |
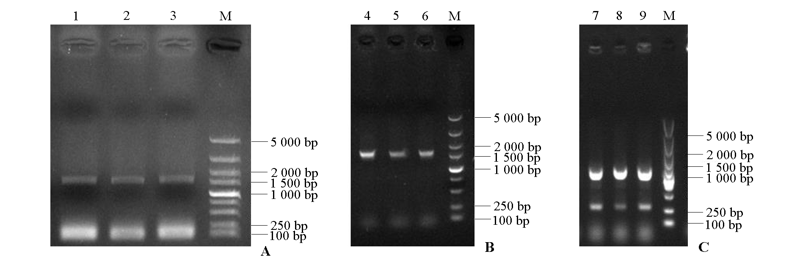
图4 RhRCA1启动子PCR扩增(A)和表达载体PCR鉴定(B、C) 1 ~ 3:RhRCA1启动子片段;4 ~ 6:prRCA1::GUS载体;7 ~ 9:prRCA1::LUC载体;M:DL5000 marker。
Fig. 4 PCR amplification of RhRCA1 promotor(A)and PCR detection of recombinant plasmids(B,C) 1 -3:fragments of promoter of RhRCA1;4-6:prRCA1::GUS;7-9:prRCA1::LUC;M:DL5000 marker.
| 类型 Type | 调控元件 Regulated element | 基序 Motif sequence | 数量 Amount | 生物学功能 Biological function |
|---|---|---|---|---|
| 非生物胁迫响应元件 Abiotic stress responsive element | ARE | AAACCA | 1 | 厌氧诱导必需的调控元件 cis-acting regulatory element essential for the anaerobic induction |
| TC-rich repeats | GTTTTCTTAC | 1 | 防御和胁迫响应元件cis-acting element involved in defense and stress responsiveness | |
| ABRE | ACGTG | 5 | 脱落酸响应元件cis-acting element involved in the abscisic acid responsiveness | |
| RGAANNTTC | GGGAGTTTC | 1 | HSF1的结合位点 HSF1 binding site | |
| CCAAT-box | CCAAT | 2 | 热激蛋白基因表达元件cis-element for heat shock protein gene expression | |
| 组织特异性相关元件 Tissue-specific element | CACTFTPPCA1 | CACT | 20 | 叶肉特异性表达元件cis-regulatory element for mesophyll-specific gene expression |
| TGACGTVMAMY | TGACGT | 2 | 子叶特异性表达元件cis-element for Expression in cotyledons of germinated seeds | |
| RBCSCONSENSUS | AATCAA | 1 | 光调节和叶特异性表达元件cis-element for light-regulated and leaf-specific expression | |
| 光响应元件 Light responsive element | Box 4 | ATTAAT | 4 | 参与部分光响应保守DNA组件 Part of a conserved DNA module involved in light responsiveness |
| G-box | CACGTC/ACACGTGGC/ TGACACGTGGCTCT | 5 | 参与光反应的元件 cis-acting regulatory element involved in light responsiveness | |
| AE-box | AGAAACTT | 1 | 部分光响应组件 Part of a module for light response | |
| 基础元件 Basal element | CAAT-box | CAAT/TGCCAAC | 28 | 启动子和增强子调控元件 Common cis-acting element in promoter and enhancer regions |
| TATA-box | TATA/TACAAAA | 11 | 转录起始位点 Transcription initiation site | |
| 其他 Others | TGACG-motif | TGACG | 5 | 参与茉莉酸甲酯诱导表达cis-acting regulatory element involved in the MeJA-responsiveness |
表2 RhRCA1启动子的部分顺式作用元件
Table 2 Part of cis-acting elements in promoter of RhRCA1
| 类型 Type | 调控元件 Regulated element | 基序 Motif sequence | 数量 Amount | 生物学功能 Biological function |
|---|---|---|---|---|
| 非生物胁迫响应元件 Abiotic stress responsive element | ARE | AAACCA | 1 | 厌氧诱导必需的调控元件 cis-acting regulatory element essential for the anaerobic induction |
| TC-rich repeats | GTTTTCTTAC | 1 | 防御和胁迫响应元件cis-acting element involved in defense and stress responsiveness | |
| ABRE | ACGTG | 5 | 脱落酸响应元件cis-acting element involved in the abscisic acid responsiveness | |
| RGAANNTTC | GGGAGTTTC | 1 | HSF1的结合位点 HSF1 binding site | |
| CCAAT-box | CCAAT | 2 | 热激蛋白基因表达元件cis-element for heat shock protein gene expression | |
| 组织特异性相关元件 Tissue-specific element | CACTFTPPCA1 | CACT | 20 | 叶肉特异性表达元件cis-regulatory element for mesophyll-specific gene expression |
| TGACGTVMAMY | TGACGT | 2 | 子叶特异性表达元件cis-element for Expression in cotyledons of germinated seeds | |
| RBCSCONSENSUS | AATCAA | 1 | 光调节和叶特异性表达元件cis-element for light-regulated and leaf-specific expression | |
| 光响应元件 Light responsive element | Box 4 | ATTAAT | 4 | 参与部分光响应保守DNA组件 Part of a conserved DNA module involved in light responsiveness |
| G-box | CACGTC/ACACGTGGC/ TGACACGTGGCTCT | 5 | 参与光反应的元件 cis-acting regulatory element involved in light responsiveness | |
| AE-box | AGAAACTT | 1 | 部分光响应组件 Part of a module for light response | |
| 基础元件 Basal element | CAAT-box | CAAT/TGCCAAC | 28 | 启动子和增强子调控元件 Common cis-acting element in promoter and enhancer regions |
| TATA-box | TATA/TACAAAA | 11 | 转录起始位点 Transcription initiation site | |
| 其他 Others | TGACG-motif | TGACG | 5 | 参与茉莉酸甲酯诱导表达cis-acting regulatory element involved in the MeJA-responsiveness |
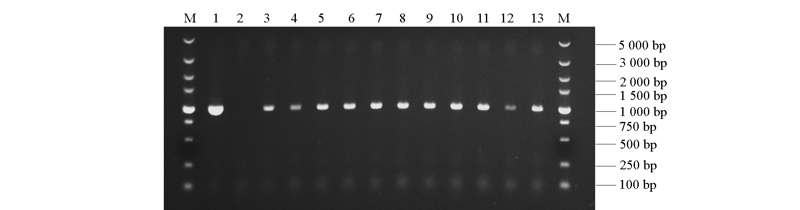
图6 转基因拟南芥PCR检测 1:prRCA1::GUS质粒(阳性对照);2:野生型植株(阴性对照)3 ~ 13:转基因植株;M:DL5000 marker。
Fig. 6 PCR detection of transgenic Arabidopsis 1:prRCA1::GUS(positive control)plasmid;2:Non-transgenic plant(negative control);3-13:Transgenic plant;M:DL5000 marker.
| [1] |
Attaran E, Major I, Cruz J, Rosa B, Koo A, Chen J, Kramer D, He S, Howe G. 2014. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiology, 165 (3):1302-1314.
pmid: 24820026 |
| [2] |
Berry J, Bjönkman O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology, 31 (1):491-543.
doi: 10.1146/annurev.pp.31.060180.002423 URL |
| [3] |
Bota J, Medrano H, Flexas J. 2004. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytologist, 162 (3):671-681.
doi: 10.1111/nph.2004.162.issue-3 URL |
| [4] |
Bracher A, Spencer M, Hartl F, Hayer-Hartl M. 2017. Biogenesis and metabolic maintenance of Rubisco. Annual Review of Plant Biology, 68 (1):29-60.
doi: 10.1146/annurev-arplant-043015-111633 URL |
| [5] |
Chao M N, Yin Z T, Hao D R, Zhang J Y, Song H N, Ning A L, Xu X M, Yu D Y. 2014. Variation in Rubisco activase(RCAβ)gene promoters and expression in soybean[Glycine max(L.)Merr.]. Journal of Experimental Botany, 65 (1):47-59.
doi: 10.1093/jxb/ert346 URL |
| [6] |
Chen C X, Hussain N, Wang Y R, Li M T, Liu T, Qin M Z, Ma N, Gao J P, Sun X M. 2020. An ethylene-inhibited NF-YC transcription factor RhNF-YC 9 regulates petal expansion in rose. Horticultural Plant Journal, 6 (6):419-427.
doi: 10.1016/j.hpj.2020.11.007 URL |
| [7] | Crafts-Brandner S J, Salvucci M E. 2000. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO 2. Proceedings of the National Academy of Sciences of the United States of America, 97 (24):13430-13435. |
| [8] |
Demirevska-Kepova K, Holzer R, Simova-Stoilova L, Feller U. 2005. Heat stress effects on Ribulose-1,5-bisphosphate carboxylase/oxygenase,Rubisco binding protein and Rubisco activase in wheat leaves. Biologia Plantarum, 49 (4):521-525.
doi: 10.1007/s10535-005-0045-2 URL |
| [9] | Fukayama H, Abe R, Uchida N. 2010. SDS-dependent proteases induced by ABA and its relation to Rubisco and Rubisco activase contents in rice leaves. Plant Physiology & Biochemistry, 48 (10):808-812. |
| [10] |
Haralampidis K, Milioni D, Rigas S, Hatzopoulos P. 2002. Combinatorial interaction of cis elements specifies the expression of the Arabidopsis AtHsp90-1 gene. Plant Physiology, 129 (3):1138-1149.
doi: 10.1104/pp.004044 URL |
| [11] | He Ya-fei, Li Xia, Xie Yin-feng. 2017. Advances in molecular mechanisms of Rubisco and Rubisco activase. Molecular Plant Breeding, 15 (8):3295-3301. (in Chinese) |
| 何亚飞, 李霞, 谢寅峰. 2017. Rubisco与Rubisco活化酶的分子机理研究进展. 分子植物育种, 15 (8):3295-3301. | |
| [12] |
Jefferson R A, Kavanagh T A, Bevan M W. 1987. GUS fusions:beta-glucuronidase as a sensitive and versaile gene fusion marker in higher plants. The EMBO Journal, 6 (13):3901-3907.
doi: 10.1002/embj.1987.6.issue-13 URL |
| [13] | Jensen R G. 2000. Activation of Rubisco regulates photosynthesis at high temperature and CO2. Proceedings of the National Academy of Sciences of the United States of America, 97 (24):12937-12939. |
| [14] |
Jiao Yu-ling, Sun L O, Wang Deng-xing. 2007. Light-regulated transcriptional networks in higher plants. Nature Reviews Genetics, 8 (3):217-230.
doi: 10.1038/nrg2049 URL |
| [15] |
Kurek I, Chang T K, Bertain S M, Madrigal A, Lu Liu, Lassner W M, Zhu Gen-hai. 2007. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. The Plant Cell, 19 (10):3230-3241.
doi: 10.1105/tpc.107.054171 URL |
| [16] | Liu Yu, Song Xi-qiang, Shi You-hai. 2018. Physiological responses comparison of Rhododendron hainanense and Rhododendron mucronatum(Blume) G. Don under high temperature stress. Molecular Plant Breeding, 16 (17):5827-5834. (in Chinese) |
| 刘宇, 宋希强, 史佑海. 2018. 高温胁迫下海南杜鹃和白花杜鹃的生理响应比较分析. 分子植物育种, 16 (17):5827-5834. | |
| [17] |
Liu Zong-rang, Taub C C, McClung C R. 1996. Identification of an Arabidopsis thaliana Ribulose-1,5-Bisphosphate Carboxylase Oxygenase Activase(RCA)minimal promoter regulated by light and the circadian clock. Plant Physiology, 112 (1):43-51.
doi: 10.1104/pp.112.1.43 URL |
| [18] | Makino A, Mae T, Ohira K. 1983. Purification and storage of Ribulose 1,5-bisphosphate carboxylase from rice leaves. Plant and Cell Physiology, 24 (6):1169-1173. |
| [19] | Neuwald A F, Aravind L, Spouge J L, Koonin E. 1999. AAA+:a class of chaperone-like ATPases associated with the assembly,operation,and disassembly of protein complexes. Genome Research, 9 (1):27-43. |
| [20] |
Orozco B M, Orgen W L. 1993. Localization of light-inducible and tissue-specific regions of the spinach ribulose bisphosphate carboxylase oxygenase(Rubisco)activase promoter in transgenic tobacco plants. Plant Molecular Biology, 23 (6):1129-1138.
doi: 10.1007/BF00042347 URL |
| [21] |
Pelham H R. 1982. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell, 30 (2):517-528.
pmid: 6814763 |
| [22] |
Pernille K, Vollaard N B J, Gustafsson T, Gallagher I J, Sundberg C J, Tuomo R, Britton S L, Claude B, Koch L G, Timmons J A. 2010. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. Journal of Applied Physiology, 110 (1):46-59.
doi: 10.1152/japplphysiol.00634.2010 URL |
| [23] | Ponjavic J, Lenhard B, Kai C, Kawai J, Carninci P, Hayashizaki Y, Sandelin A. 2006. Transcriptional and structural impact of TATA-initiation site spacing in mammalian core promoters. Genome Biology, 7 (8):5-19. |
| [24] |
Portis A R. 2003. Rubisco activase-Rubisco's catalytic chaperone. Photosynthesis Research, 75 (1):11-27.
doi: 10.1023/A:1022458108678 URL |
| [25] |
Qu D, Song Y, Li W M, Pei X W, Wang Z X, Jia S R, Zhang Y Q. 2011. Isolation and characterization of the organ-specific and light-inducible promoter of the gene encoding Rubisco activase in potato(Solanum tuberosum). Genetics and Molecular Research, 10 (2):621-631.
doi: 10.4238/vol10-2gmr1088 pmid: 21491372 |
| [26] | Rieping M, Schöffl F. 1992. Synergistic effect of upstream sequences,CCAAT box elements,and HSE sequences for enhanced expression of chimaeric heat shock genes in transgenic tobacco. MGG Molecular & General Genetics, 231 (2):226-232. |
| [27] |
Salvucci M E. 2008. Association of Rubisco activase with chaperonin-60β:a possible mechanism for protecting photosynthesis during heat stress. Journal of Experimental Botany, 59 (7):1923-1933.
doi: 10.1093/jxb/erm343 pmid: 18353762 |
| [28] |
Scafaro A P, Haynes P A, Atwell B J. 2009. Physiological and molecular changes in Oryza meridionalis Ng.,a heat-tolerant species of wild rice. Journal of Experimental Botany, 61 (1):191-201.
doi: 10.1093/jxb/erp294 URL |
| [29] |
Shelton A M, Zhao J Z, Roush R T. 2002. Economic,ecological,food safety,and social consequences of the development of Bt transgenic plants. Annual Review of Entomology, 47:845-881.
doi: 10.1146/annurev.ento.47.091201.145309 URL |
| [30] | Shi You-hai, Li Shao-peng, Liang Wei-hong, Song Xi-qiang, Tan Jin-hong. 2010. Germplasm resourses of Rhododendron in Hainan. Chinese Journal of Tropical Crops, 31 (4):551-555. (in Chinese) |
| 史佑海, 李绍鹏, 梁伟红, 宋希强, 谭金红. 2010. 海南野生杜鹃花属植物种质资源调查研究. 热带作物学报, 31 (4):551-555. | |
| [31] |
Wang Xiu-yun, Li Zheng, Liu Bing, Zhou Hong, Elmongy M S, Xia Yi-ping. 2020. Combined proteome and transcriptome analysis of heat-primed azalea reveals new insights into plant heat acclimation memory. Frontiers in Plant Science, 11:1278. doi: 10.3389/fpls.2020.01278.
doi: 10.3389/fpls.2020.01278 URL |
| [32] |
Xu Dong-qing, Li Ji-gang, Gangappa S N, Chamari H, Lin Fang, Mats A X, Jiang Yan, Wang Deng-xing, Magnus H. 2014. Convergence of light and ABA signaling on the ABI5 promoter. PLoS Genetics, 10 (2):e1004197.
doi: 10.1371/journal.pgen.1004197 URL |
| [33] |
Yang Zhi-pan, Lu Qing-tao, Wen Xiao-gang, Chen Fan, Lu Cong-ming. 2012. Functional analysis of the rice rubisco activase promoter in transgenic Arabidopsis. Biochemical and Biophysical Research Communications, 418 (3):565-570.
doi: 10.1016/j.bbrc.2012.01.073 URL |
| [34] |
Zhao Ying, Yu Wen-gang, Hu Xiang-yu, Shi You-hai, Liu Yu, Zhong Yun-fang, Wang Peng, Deng Shu-ya, Niu Jun, Yu Xu-dong. 2018. Physiological and transcriptomic analysis revealed the involvement of crucial factors in heat stress response of Rhododendron hainanense. Gene, 660:109-119.
doi: S0378-1119(18)30320-2 pmid: 29604462 |
| [35] | Zou Cheng, Sun Ke-lian, Mackaluso J D, Seddon A E, Jin Rong, Thomashow M F, Shin-Han S. 2011. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America, 108 (36):14992-14997. |
| [1] | 于婷婷, 李 欢, 宁源生, 宋建飞, 彭璐琳, 贾竣淇, 张玮玮, 杨洪强. 苹果GRAS全基因组鉴定及其对生长素的响应分析[J]. 园艺学报, 2023, 50(2): 397-409. |
| [2] | 袁馨, 徐云鹤, 张雨培, 单楠, 陈楚英, 万春鹏, 开文斌, 翟夏琬, 陈金印, 甘增宇. 猕猴桃后熟过程中ABA响应结合因子AcAREB1调控AcGH3.1的表达[J]. 园艺学报, 2023, 50(1): 53-64. |
| [3] | 郑一威, 沃科军, 蒋宝鑫, 周若一, 谢晓鸿, . 杜鹃花新品种‘甬紫蝶’[J]. 园艺学报, 2022, 49(S2): 241-242. |
| [4] | 王 华, 王 莲, 贾素琦, 赵姝玮, 张水明, 郁书俊. 杜鹃花新品种‘皖娇’[J]. 园艺学报, 2022, 49(S2): 243-244. |
| [5] | 田晓玲, 马永鹏, . 高山杜鹃新品种‘流光溢彩’[J]. 园艺学报, 2022, 49(S2): 245-246. |
| [6] | 张春英, 夏 溪, 苏 鸣, 张 杰, 龚 睿. 杜鹃花新品种‘胭脂’[J]. 园艺学报, 2022, 49(S1): 167-168. |
| [7] | 林元秘, 朱文姣, 陈敏, 薛春梅, 晋芳宇, 朱羽平, 蒋欣玥, 叶凌峰, 倪姝南伶, 杨清. miR396b负调控茄子对黄萎病的防御反应[J]. 园艺学报, 2022, 49(8): 1713-1722. |
| [8] | 赵建荣, 杨圆, 秦改花, 刘春燕, 于晴, 贾波涛, 苏颖, 曹榛, 黎积誉. 石榴HAK/KUP/KT家族基因鉴定及钾转运功能分析[J]. 园艺学报, 2022, 49(4): 758-768. |
| [9] | 王丹, 王谧, 刘军, 周晓慧, 刘松瑜, 杨艳, 庄勇. 茄子U6启动子克隆及CRISPR/Cas9介导的基因编辑体系建立[J]. 园艺学报, 2022, 49(4): 791-800. |
| [10] | 相立, 赵蕾, 王玫, 吕毅, 王艳芳, 沈向, 陈学森, 尹承苗, 毛志泉. 苹果MdWRKY74的克隆和功能分析[J]. 园艺学报, 2022, 49(3): 482-492. |
| [11] | 赵晖, 耿兴敏, 王露露, 许世达. 乙烯在杜鹃花耐热机制中的作用研究[J]. 园艺学报, 2022, 49(3): 561-570. |
| [12] | 宋放, 李子璇, 王策, 王志静, 何利刚, 蒋迎春, 吴黎明, 白福玺. 柑橘菌根信号受体蛋白基因LYK2的克隆及功能分析[J]. 园艺学报, 2022, 49(2): 281-292. |
| [13] | 孙威, 孙世宇, 陈一然, 王聿晗, 张艳, 鞠志刚, 乙引. 马缨杜鹃查尔酮异构酶基因RdCHI1的克隆与功能解析[J]. 园艺学报, 2022, 49(11): 2407-2418. |
| [14] | 黄仁维, 任迎虹, 祁伟亮, 曾睿, 刘欣宇, 邓彬艳. 桑树MaERF105-Like的克隆及其在干旱胁迫下的表达分析[J]. 园艺学报, 2022, 49(11): 2439-2448. |
| [15] | 杨天宸, 陈晓童, 吕可, 张荻. 百子莲脱水素基因ApSK3对逆境与激素信号的应答模式与调控机制[J]. 园艺学报, 2021, 48(8): 1565-1578. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司