
园艺学报 ›› 2021, Vol. 48 ›› Issue (3): 505-517.doi: 10.16420/j.issn.0513-353x.2020-0520
收稿日期:2020-07-08
出版日期:2021-03-25
发布日期:2021-04-02
通讯作者:
曹家树
E-mail:jshcao@ziu.edu.cn
基金资助:Received:2020-07-08
Online:2021-03-25
Published:2021-04-02
Contact:
CAO Jiashu
E-mail:jshcao@ziu.edu.cn
摘要:
在从白菜‘矮脚黄’自交系Bcajh97-01中克隆获得多聚半乳糖醛酸酶基因BcPG17及其启动子序列的基础上,采用qRT-PCR、原位杂交和启动子融合表达载体瞬时转化技术,分析了该基因的表达特征。BcPG17的DNA全长为2 105 bp,包含9个外显子和8个内含子。ORF序列长度为1 344 bp,编码447个氨基酸残基。预测的编码蛋白的相对分子量为48.61 kD,理论等电点为8.39,含有1个跨膜结构域,N末端具有信号肽序列,具有被分泌到细胞膜外起作用的特征;其氨基酸序列具有PG蛋白的4个典型结构域,与十字花科芸薹属的其他物种亲缘关系较近。通过qRT-PCR分析发现,BcPG17在开花期花茎中的表达水平最高,且在花粉发育的四分体时期和单核小孢子时期有表达。原位杂交试验结果也显示,BcPG17在开花期花茎的所有组织中均存在强烈的表达信号。通过对克隆获得的1 442 bp的BcPG17启动子序列分析,发现该序列含有与分生组织表达有关的顺式作用元件1个、与植物激素响应相关的作用基序和元件多个,以及花药特异基序4个和绒毡层降解延迟蛋白(TDR)结合位点2个;能够启动GUS信号在开花期花茎的第2节间和节处强烈表达,在花发育中期的花药中也存在明显的GUS信号。上述结果表明,BcPG17可能通过与植物激素代谢相关蛋白相互作用调控花茎的伸长,受到绒毡层降解相关蛋白的调控参与花粉的发育。
中图分类号:
吕美玲, 曹家树. 白菜花茎发育相关基因BcPG17的表达特征分析[J]. 园艺学报, 2021, 48(3): 505-517.
LÜ Meiling, CAO Jiashu. Expression Characteristic Analysis of the BcPG17,a Gene Related to Inflorescence Stem Development of Brassica campestris[J]. Acta Horticulturae Sinica, 2021, 48(3): 505-517.
| 物种(基因名称) Species(gene name) | GenBank登录号 GenBank No. | |||
|---|---|---|---|---|
| 拟南芥Arabidopsis thaliana(PGA3) | NP_187439.1 | |||
| 拟南芥Arabidopsis thaliana(PGA2) | CAA51692.1 | |||
| 白菜Brassica campestris(BcMF2) | ABW24665.1 | |||
| 白菜Brassica campestris(BcMF6) | ACP74159.1 | |||
| 白菜Brassica campestris(BcMF9) | ABN13878.1 | |||
| 白菜Brassica campestris(BcMF16) | ADJ68232.1 | |||
| 油菜Brassica napus(RDPG) | CAA65072.1 | |||
| 油菜Brassica napus(PGAZ) | CAC05658.1 | |||
| 甜瓜Cucumis melo(MPG2) | AAC26511.1 | |||
| 陆地棉Gossypium hirsutum(G9) | AAA82167.1 | |||
| 荔枝Litchi chinensisv(LcPG1) | AFW04075.1 | |||
| 番茄Solanum lycopersicum(TAPG1) | AAC28903.1 | |||
| 番茄Solanum lycopersicum(TAPG2) | AAC28904.1 | |||
| 烟草Nicotiana tabacum(PG1) | Q05967.1 | |||
| 桃Prunus persica(PRF5) | CAA54150.1 | |||
表1 多序列比对分析所用的PG基因信息
Table 1 Details of the PG genes used in the multiple alignment analysis
| 物种(基因名称) Species(gene name) | GenBank登录号 GenBank No. | |||
|---|---|---|---|---|
| 拟南芥Arabidopsis thaliana(PGA3) | NP_187439.1 | |||
| 拟南芥Arabidopsis thaliana(PGA2) | CAA51692.1 | |||
| 白菜Brassica campestris(BcMF2) | ABW24665.1 | |||
| 白菜Brassica campestris(BcMF6) | ACP74159.1 | |||
| 白菜Brassica campestris(BcMF9) | ABN13878.1 | |||
| 白菜Brassica campestris(BcMF16) | ADJ68232.1 | |||
| 油菜Brassica napus(RDPG) | CAA65072.1 | |||
| 油菜Brassica napus(PGAZ) | CAC05658.1 | |||
| 甜瓜Cucumis melo(MPG2) | AAC26511.1 | |||
| 陆地棉Gossypium hirsutum(G9) | AAA82167.1 | |||
| 荔枝Litchi chinensisv(LcPG1) | AFW04075.1 | |||
| 番茄Solanum lycopersicum(TAPG1) | AAC28903.1 | |||
| 番茄Solanum lycopersicum(TAPG2) | AAC28904.1 | |||
| 烟草Nicotiana tabacum(PG1) | Q05967.1 | |||
| 桃Prunus persica(PRF5) | CAA54150.1 | |||
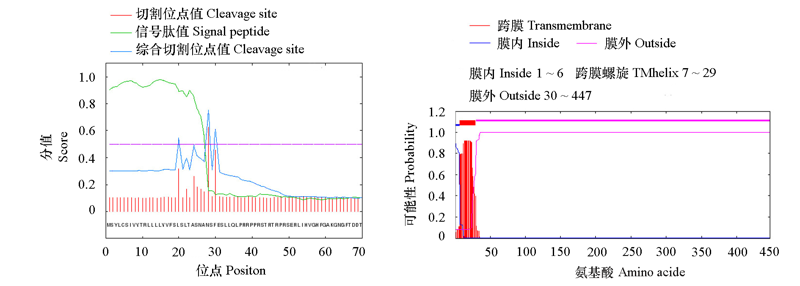
图2 BcPG17的信号肽(A)和跨膜结构(B)的预测 信号肽与成熟肽之间的分割位点可能位于第27和第28位氨基酸残基之间。
Fig. 2 Signal peptide(A)and transmembrane helices(B)prediction of BcPG17 The most likely cleavage site of signal peptide is between the position of 27 and 28.
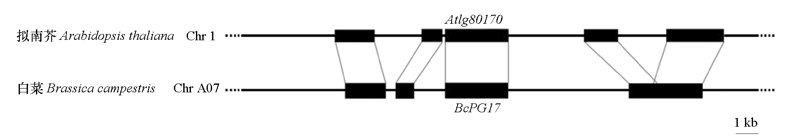
图4 BcPG17及其在拟南芥中的同源基因At1g80170所在染色体区段的共线性分析
Fig. 4 Collinearity analysis of the chromosome segments where BcPG17 and its homologous gene At1g80170 in Arabidopsis thaliana are located
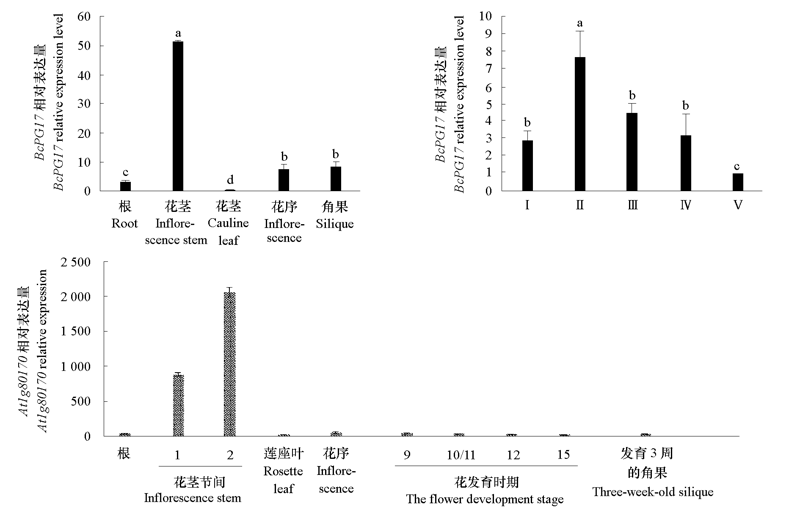
图5 BcPG17的表达特征和拟南芥数据库中At1g80170 ATH1基因芯片数据分析 Ⅰ~ Ⅴ分别代表花粉母细胞时期、四分体时期、单核小孢子时期、双核小孢子时期和三核成熟花粉期的花蕾。
Fig. 5 Analysis of the expression pattern of BcPG17 and the ATH1 GeneChip data of At1g80170 from Arabidopsis database Ⅰ ~ Ⅴ indicate the buds of pollen mother cell stage,tetrad stage,uninucleate microspore stage,binucleate microspore stage and mature pollen respectively.
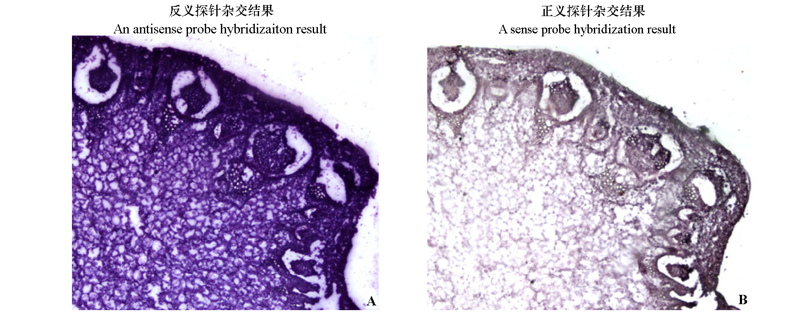
图6 BcPG17在白菜开花期花茎横切片的原位杂交结果
Fig. 6 The in situ hybridization result of BcPG17 in cross-section inflorescence stem of Brassica campestris during flowering stage
| 顺式作用元件 cis-acting element | 序列 Sequence | 功能 Function | 数量 Number |
|---|---|---|---|
| CAAT-box | CAAAT | 启动子和增强子区域常见的顺式作用元件 Common cis-acting element in promoter and enhancer regions | 8 |
| TATA-box | TATAAAT/TATA | 转录起始位点周围-30左右的核心启动子元件 Core promoter element around-30 of transcription start | 44 |
| TCA-element | CCATCTTTTT | 水杨酸响应相关的顺式作用元件 cis-acting element involved in salicylic acid responsiveness | 2 |
| ABRE | ACGTG | 脱落酸响应相关的顺式作用元件 cis-acting element involved in the abscisic acid responsiveness | 1 |
| TGACG/CGTCA-motif | TGACG/CGTCA | 茉莉酸甲酯响应相关的顺式作用调节元件 cis-acting regulatory element involved in the MeJA-responsiveness | 4 |
| GARE-motif | TCTGTTG | 赤霉素响应元件 Gibberellin-responsive element | 1 |
| CAT-box | GCCACT | 与分生组织表达有关的顺式调控元件 cis-acting regulatory element related to meristem expression | 1 |
| ARE | AAACCA | 厌氧诱导的关键顺式作用调节元件 cis-acting regulatory element essential for the anaerobic induction | 2 |
| G-Box | CACGTT | 参与光响应的顺式作用调节元件 cis-acting regulatory element involved in light responsiveness | 1 |
| Box 4 | ATTAAT | 光响应相关的保守DNA模块的一部分 Part of a conserved DNA module involved in light responsiveness | 4 |
| Gap-box | CAAATGAA(A/G)A | 光响应元件的一部分 Part of a light responsive element | 1 |
| TCT-motif | TCTTAC | 光响应元件的一部分 Part of a light responsive element | 1 |
| AT1-motif | AATTATTTTTTATT | 光响应元件的一部分 Part of a light responsive element | 1 |
| AE-box | AGAAACAA | 光响应元件的一部分 Part of a light responsive element | 1 |
| GTGA-motif | GTGA | 花药特异基序 Anther specific motif | 4 |
| E-box | CATTG | 绒毡层降解延迟蛋白(TDR)结合位点 Tapetum Degeneration Retardation(TDR)binding site | 2 |
表2 BcPG17启动子序列中的主要顺式作用元件
Table 2 The major cis-acting elements of BcPG17 promoter
| 顺式作用元件 cis-acting element | 序列 Sequence | 功能 Function | 数量 Number |
|---|---|---|---|
| CAAT-box | CAAAT | 启动子和增强子区域常见的顺式作用元件 Common cis-acting element in promoter and enhancer regions | 8 |
| TATA-box | TATAAAT/TATA | 转录起始位点周围-30左右的核心启动子元件 Core promoter element around-30 of transcription start | 44 |
| TCA-element | CCATCTTTTT | 水杨酸响应相关的顺式作用元件 cis-acting element involved in salicylic acid responsiveness | 2 |
| ABRE | ACGTG | 脱落酸响应相关的顺式作用元件 cis-acting element involved in the abscisic acid responsiveness | 1 |
| TGACG/CGTCA-motif | TGACG/CGTCA | 茉莉酸甲酯响应相关的顺式作用调节元件 cis-acting regulatory element involved in the MeJA-responsiveness | 4 |
| GARE-motif | TCTGTTG | 赤霉素响应元件 Gibberellin-responsive element | 1 |
| CAT-box | GCCACT | 与分生组织表达有关的顺式调控元件 cis-acting regulatory element related to meristem expression | 1 |
| ARE | AAACCA | 厌氧诱导的关键顺式作用调节元件 cis-acting regulatory element essential for the anaerobic induction | 2 |
| G-Box | CACGTT | 参与光响应的顺式作用调节元件 cis-acting regulatory element involved in light responsiveness | 1 |
| Box 4 | ATTAAT | 光响应相关的保守DNA模块的一部分 Part of a conserved DNA module involved in light responsiveness | 4 |
| Gap-box | CAAATGAA(A/G)A | 光响应元件的一部分 Part of a light responsive element | 1 |
| TCT-motif | TCTTAC | 光响应元件的一部分 Part of a light responsive element | 1 |
| AT1-motif | AATTATTTTTTATT | 光响应元件的一部分 Part of a light responsive element | 1 |
| AE-box | AGAAACAA | 光响应元件的一部分 Part of a light responsive element | 1 |
| GTGA-motif | GTGA | 花药特异基序 Anther specific motif | 4 |
| E-box | CATTG | 绒毡层降解延迟蛋白(TDR)结合位点 Tapetum Degeneration Retardation(TDR)binding site | 2 |
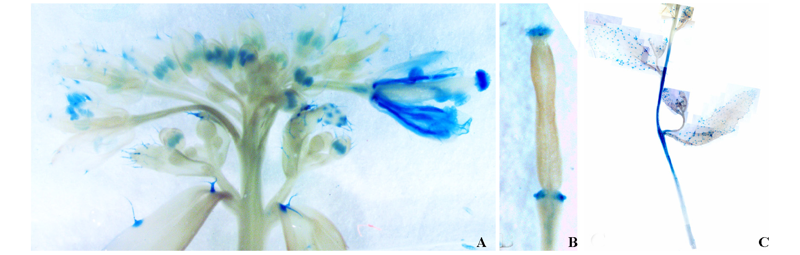
图9 转化pBGWFS7.0-proBcPG17: GUS-GFP载体的T3代拟南芥阳性植株的GUS组织化学分析
Fig. 9 The histochemical GUS assays of T3 Arabidopsis thaliana plants transformed with pBGWFS7.0-proBcPG17:GUS-GFP vector
| [1] |
Allen G C, Flores-Vergara M A, Krasnyanski S, Kumar S, Thompson W F. 2006. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nature Protocols, 1:2320-2325.
pmid: 17406474 |
| [2] | Bell A D, Bryan A. 2008. Plant form:an illustrated guide to flowering plant morphology. Portland: Timber Press. |
| [3] |
Camejo D, Martí M C, Jiménez A, Cabrera J C, Olmos E, Sevilla F. 2011. Effect of oligogalacturonides on root length,extracellular alkalinization and O-2 (-)-accumulation in alfalfa. Journal of Plant Physiology, 168:566-575.
doi: 10.1016/j.jplph.2010.09.012 pmid: 21074893 |
| [4] |
Carpita N C, Gibeaut D M. 1993. Structural models of primary-cell walls in flowering plants-consistency of molecular-structure with the physical-properties of the walls during growth. Plant Journal, 3:1-30.
pmid: 8401598 |
| [5] |
Cassab G I. 1998. Plant cell wall proteins. Annual Review of Plant Physiology and Plant Molecular Biology, 49:281-309.
doi: 10.1146/annurev.arplant.49.1.281 URL |
| [6] |
Chen L, Tu Z M, Hussain J, Cong L, Yan Y J, Jin L, Yang G X, He G Y. 2010. Isolation and heterologous transformation analysis of a pollen-specific promoter from wheat(Triticum aestivum L.). Molecular Biology Reports, 37:737-744.
doi: 10.1007/s11033-009-9582-7 URL |
| [7] |
Dayan J, Voronin N, Gong F, Sun T P, Hedden P, Fromm H, Aloni A. 2012. Leaf-induced gibberellin signaling is essential for internode elongation,cambial activity,and fiber differentiation in tobacco stems. The Plant Cell, 24 (1):66-79.
doi: 10.1105/tpc.111.093096 URL |
| [8] | Demura T, Tashiro G, Horiguchi G, Kishimoto N, Kubo M, Matsuoka N, Minami A, Nagata-Hiwatashi M, Nakamura K, Okamura Y, Sassa N, Suzuki S, Yazaki J, Kikuchi S, Fukuda H. 2002. Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells. Proceedings of the National Academy of Sciences of the United States of America, 99:15794-15799. |
| [9] |
Falasca G, Capitani F, Della Rovere F, Zaghi D, Franchin C, Biondi S, Altamura M M. 2008. Oligogalacturonides enhance cytokinin-induced vegetative shoot formation in tobacco explants,inhibit polyamine biosynthetic gene expression,and promote long-term remobilisation of cell calcium. Planta, 227:835-852.
doi: 10.1007/s00425-007-0660-6 URL |
| [10] | Gifford E M, Foster A S. 1989. Morphology and evolution of vascular plants. New York: Freeman. |
| [11] |
Gomez-Ariza J, Brambilla V, Vicentini G, Landini M, Cerise M, Carrera E, Shrestha R, Chiozzotto R, Galbiati F, Caporali E, López Díaz I, Fornara F. 2019. A transcription factor coordinating internode elongation and photoperiodic signals in rice. Nature Plants, 5:358-362.
doi: 10.1038/s41477-019-0401-4 |
| [12] |
Gómez-Mena C, Sablowski R. 2008. Arabidopsis thaliana HOMEOBOX GENE1 establishes the basal boundaries of shoot organs and controls stem growth. Plant Cell, 20:2059-2072.
doi: 10.1105/tpc.108.059188 URL |
| [13] |
Hadfield K A, Bennett A B. 1998. Polygalacturonases:many genes in search of a function. Plant Physiology, 117 (2):337-343.
doi: 10.1104/pp.117.2.337 URL |
| [14] | Harrison C J, Morris J L. 2018. The origin and early evolution of vascular plant shoots and leaves. Philosophical Transactions of the Royal Society of London. SeriesB:Biological Sciences, 373:20160496. |
| [15] |
Hepworth S R, Pautot V A. 2015. Beyond the divide:boundaries for patterning and stem cell regulation in plants. Frontiers in Plant Science, 6:1052.
doi: 10.3389/fpls.2015.01052 pmid: 26697027 |
| [16] |
Huang L, Cao J S, Zhang A H, Ye Y Q, Zhang Y C, Liu T T. 2009a. The polygalacturonase gene BcMF2 from Brassica campestris is associated with intine development. Journal of Experimental Botany, 60:301-313.
doi: 10.1093/jxb/ern295 URL |
| [17] |
Huang L, Ye Y, Zhang Y, Zhang A, Liu T, Cao J. 2009b. BcMF9,a novel polygalacturonase gene, is required for both Brassica campestris intine and exine formation. Annals of Botany, 104:1339-1351.
doi: 10.1093/aob/mcp244 URL |
| [18] |
Huang L, Zhao X F, Liu T T, Dong H, Cao J S. 2010. Developmental characteristics of floral organs and pollen of Chinese cabbage( Brassica campestris L. ssp. chinensis). Plant Systematics and Evolution, 286:103-115.
doi: 10.1007/s00606-010-0283-4 URL |
| [19] |
Hwang H J, Kim H, Jeong Y M, Choi M Y, Lee S Y, Kim S G. 2011. Overexpression of EVE1,a novel ubiquitin family protein,arrests inflorescence stem development in Arabidopsis. Journal of Experimental Botany, 62:4571-458.
doi: 10.1093/jxb/err168 URL |
| [20] |
Imoto K, Yokoyama R, Nishitani K. 2005. Comprehensive approach to genes involved in cell wall modifications in Arabidopsis thaliana. Plant Molecular Biology, 58 (2):177-192.
doi: 10.1007/s11103-005-5344-7 URL |
| [21] |
Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K. 2007. Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. The Plant Cell, 19:3549-3562.
doi: 10.1105/tpc.107.054536 URL |
| [22] |
Kaplan D R, Hagemann W. 1991. The relationship of cell and organism in vascular plants. BioScience, 41:693-703.
doi: 10.2307/1311764 URL |
| [23] |
Kemi U, Leinonen P H, Savolainen O, Kuittinen H. 2019. Inflorescence shoot elongation,but not flower primordia formation,is photoperiodically regulated in Arabidopsis lyrata. Annals of Botany, 124 (1):91-101.
doi: 10.1093/aob/mcz035 URL |
| [24] |
Khan M, Xu M, Murmu J, Tabb P, Liu Y, Storey K, McKim S M, Douglas C J, Hepworth S R. 2012. Antagonistic interaction of BLADE-ON-PETIOLE1 and 2 with BREVIPEDICELLUS and PENNYWISE regulates Arabidopsis inflorescence architecture. Plant Physiology, 158:946-960.
doi: 10.1104/pp.111.188573 URL |
| [25] |
Kim J, Shiu S H, Thoma S, Li W H, Patterson S E. 2006. Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biology, 7 (9):R87.
doi: 10.1186/gb-2006-7-9-r87 URL |
| [26] |
Koizumi K, Yokoyama R, Nishitani K. 2009. Mechanical load induces upregulation of transcripts for a set of genes implicated in secondary wall formation in the supporting tissue of Arabidopsis thaliana. Journal of Plant Research, 122:651-659.
doi: 10.1007/s10265-009-0251-7 pmid: 19582540 |
| [27] |
Liang Y, Yu Y, Shen X, Dong H, Lyu M, Xu L, Ma Z, Liu T, Cao J. 2015. Dissecting the complex molecular evolution and expression of polygalacturonase gene family in Brassica rapa ssp. chinensis. Plant Molecular Biology, 89 (6):629-646.
doi: 10.1007/s11103-015-0390-2 pmid: 26506823 |
| [28] |
Liu N N. 2019. Effects of IAA and ABA on the immature peach fruit development process. Horticultural Plant Journal, 5 (4):145-154.
doi: 10.1016/j.hpj.2019.01.005 URL |
| [29] |
Liu X H, Shangguan Y Y, Zhu J J, Lu Y Q, Han B. 2013. The rice OsLTP6 gene promoter directs anther-specific expression by a combination of positive and negative regulatory elements. Planta, 238:845-857.
doi: 10.1007/s00425-013-1934-9 URL |
| [30] |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 -∆∆CT method. Methods, 25:402-408.
pmid: 11846609 |
| [31] |
Lyu M, Liang Y, Yu Y, Ma Z, Song L, Yue X, Cao J. 2015. Identification and expression analysis of BoMF25,a novel polygalacturonase gene involved in pollen development of Brassica oleracea. Plant Reproduction, 28:121-132.
doi: 10.1007/s00497-015-0263-5 URL |
| [32] |
McKim S M. 2019. How plants grow up. Journal of Integrative Plant Biology, 61:257-277.
doi: 10.1111/jipb.v61.3 URL |
| [33] |
McKim S M. 2020. Moving on up-controlling internode growth. New Phytologist, 226 (3):672-678.
doi: 10.1111/nph.v226.3 URL |
| [34] |
Narusaka M, Shiraishi T, Iwabuchi M, Narusaka Y. 2010. The floral inoculating protocol: a simplified Arabidopsis thaliana transformation method modified from floral dipping. Plant biotechnology, 27 (4):349-351.
doi: 10.5511/plantbiotechnology.27.349 URL |
| [35] |
Ogawa M, Kay P, Wilson S, Swain S M. 2009. Arabidopsis Dehiscence Zone Polygalacturonase1( ADPG1), ADPG2,and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. The Plant Cell, 21:216-233.
doi: 10.1105/tpc.108.063768 URL |
| [36] |
Paniagua C, Pose S, Morris V J, Kirby A R, Quesada M A, Mercado J A. 2014. Fruit softening and pectin disassembly: an overview of nanostructural pectin modifications assessed by atomic force microscopy. Annals of Botany, 114:1375-1383.
doi: 10.1093/aob/mcu149 URL |
| [37] | Patil V, McDermott H I, McAllister T, Cummins M, Silva J C, Mollison E, Meikle R, Morris J, Hedley P E, Waugh R, Dockter C, Hansson M, Hansson M, McKim S M. 2019. APETALA2 control of barley internode elongation. Development, 146:dev170373. |
| [38] |
Piffanelli P, Ross J H E, Murphy D J. 1998. Biogenesis and function of the lipidic structures of pollen grains. Sexual Plant Reproduction, 11:65-80.
doi: 10.1007/s004970050122 URL |
| [39] |
Rhee S Y, Osborne E, Poindexter P D, Somerville C R. 2003. Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiology, 133:1170-1180.
doi: 10.1104/pp.103.028266 URL |
| [40] |
Rhee S Y, Somerville C R. 1998. Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant Journal, 15:79-88.
pmid: 9744097 |
| [41] |
Ridley B L, O'Neill M A, Mohnen D A. 2001. Pectins: structure,biosynthesis,and oligogalacturonide-related signaling. Phytochemistry, 57:929-967.
pmid: 11423142 |
| [42] |
Rogers H J, Bate N, Combe J, Sullivan J, Sweetman J, Swan C, Lonsdale D M, Twell D. 2001. Functional analysis of cis-regulatory elements within the promoter of the tobacco late pollen gene g10. Plant Molecular Biology, 45:577-585.
doi: 10.1023/A:1010695226241 URL |
| [43] |
Roongsattham P, Morcillo F, Jantasuriyarat C, Pizot M, Moussu S, Jayaweera D, Collin M, Gonzalez-Carranza Z H, Amblard P, Tregear J W, Tragoonrung S, Verdeil J L, Tranbarger T J. 2012. Temporal and spatial expression of polygalacturonase gene family members reveals divergent regulation during fleshy fruit ripening and abscission in the monocot species oil palm. BMC Plant Biology, 12:150.
doi: 10.1186/1471-2229-12-150 pmid: 22920238 |
| [44] |
Sachs R M. 1965. Stem elongation. Annual Review of Plant Physiology, 16:73-96.
doi: 10.1146/annurev.pp.16.060165.000445 URL |
| [45] |
Sander L, Child R, Ulvskov P, Albrechtsen M, Borkhardt B. 2001. Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape( Brassica napus)and Arabidopsis thaliana:evidence for roles in cell separation in dehiscence and abscission zones,and in style tissues during pollen tube growth. Plant Molecular Biology, 46:469-479.
doi: 10.1023/A:1010619002833 URL |
| [46] | Siedlecka A, Wiklund S, Peronne M A, Micheli F, Lesniewska J, Sethson I, Edlund U, Richard L, Sundberg B, Mellerowicz E J. 2008. Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiology, 146:554-565. |
| [47] |
Sun L, van Nocker S. 2010. Analysis of promoter activity of members of the PECTATE LYASE-LIKE( PLL)gene family in cell separation in Arabidopsis. Bmc Plant Biology, 10:152.
doi: 10.1186/1471-2229-10-152 URL |
| [48] |
Sun T P. 2008. Gibberellin metabolism, percepition and signaling pathways in Arabidopsis. Arabidopsis Book, 6:e0103.
doi: 10.1199/tab.0103 URL |
| [49] |
Tacken E, Ireland H, Gunaseelan K, Karunairetnam S, Wang D, Schultz K, Bowen J, Atkinson R G, Johnston J W, Putterill J, Hellens R P, Schaffer R J. 2010. The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE1 gene and fruit softening. Plant Physiology, 153:294-305.
doi: 10.1104/pp.109.151092 pmid: 20237022 |
| [50] |
Tanaka W, Pautler M, Jackson D, Hirano H Y. 2013. Grass meristems II:inflorescence architecture,flower development and meristem fate. Plant and Cell Physiology, 54:313-324.
doi: 10.1093/pcp/pct016 URL |
| [51] |
Teo Z W N, Song S, Wang Y Q, Liu J, Yu H. 2014. New insights into the regulation of inflorescence architecture. Trends in Plant Science, 19:158-165.
doi: 10.1016/j.tplants.2013.11.001 URL |
| [52] |
Vincken J P, Schols H A, Oomen R, McCann M C, Ulvskov P, Voragen A G J, Visser R G F. 2003. If homogalacturonan were a side chain of rhamnogalacturonan I. implications for cell wall architecture. Plant Physiology, 132:1781-1789.
doi: 10.1104/pp.103.022350 URL |
| [53] |
Willats W G T, McCartney L, Mackie W, Knox J P. 2001. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology, 47:9-27.
doi: 10.1023/A:1010662911148 URL |
| [54] |
Wils C R, Kaufmann K. 2017. Gene-regulatory networks controlling inflorescence and flower development in Arabidopsis thaliana. Biochimica et Biophysica Acta-Gene Regulatory Mechanisms, 1860:95-105.
doi: 10.1016/j.bbagrm.2016.07.014 URL |
| [55] |
Yokoyama R, Nishitani K. 2006. Identification and characterization of Arabidopsis thaliana genes involved in xylem secondary cell walls. Journal of Plant Research, 119 (3):189-194.
doi: 10.1007/s10265-006-0261-7 URL |
| [56] |
Zhang D S, Liang W Q, Yin C S, Zong J, Gu F W, Zhang D B. 2010. OsC6,encoding a lipid transfer protein,is required for postmeiotic anther development in rice. Plant Physiology, 154:149-162.
doi: 10.1104/pp.110.158865 URL |
| [1] | 赵雪艳, 王琪, 王莉, 王方圆, 王庆, 李艳. 基于比较转录组的延胡索组织差异性表达分析[J]. 园艺学报, 2023, 50(1): 177-187. |
| [2] | 韩书辉, 韩彩锋, 韩书荣, 韩彩梅, 韩 旭. 秋大白菜新品种‘胶研秋宝’[J]. 园艺学报, 2022, 49(S2): 83-84. |
| [3] | 汪维红, 张凤兰, 余阳俊, 张德双, 赵岫云, 于拴仓, 苏同兵, 李佩荣, 辛晓云. 秋大白菜新品种‘京秋1518’[J]. 园艺学报, 2022, 49(S2): 85-86. |
| [4] | 余阳俊, 汪维红, 苏同兵, 张凤兰, 张德双, 赵岫云, 于拴仓, 李佩荣, 辛晓云, 王 姣. 抗根肿病耐抽薹大白菜新品种‘京春CR3’[J]. 园艺学报, 2022, 49(S2): 87-88. |
| [5] | 王丽丽, 王 鑫, 吴海东, 温 蔷, 杨晓飞. 抗根肿病大白菜新品种‘辽白28’[J]. 园艺学报, 2022, 49(S2): 89-90. |
| [6] | 余阳俊, 苏同兵, 张凤兰, 张德双, 赵岫云, 于拴仓, 汪维红, 李佩荣, 辛晓云, 王 姣, 武长见. 紫色苗用型大白菜新品种‘京研紫快菜’[J]. 园艺学报, 2022, 49(S2): 91-92. |
| [7] | 黄 鹂, 陈财志, 余小林, 姚祥坦, 曹家树, . 早中熟普通白菜新品种‘浙大青’[J]. 园艺学报, 2022, 49(S2): 93-94. |
| [8] | 徐立功, 韩太利, 孙继峰, 杨晓东, 谭金霞. 苗用大白菜新品种‘锦绿2号’[J]. 园艺学报, 2022, 49(S1): 67-68. |
| [9] | 邵贵荣, 朱 彬, 林 晓, 曹 萍, 方 勇, 崔 田, 蒋 鹏, 林咏铭, 林 魁, 林志滔. 白菜新品种‘金品008’[J]. 园艺学报, 2022, 49(S1): 69-70. |
| [10] | 姜悦悦, 王田田, 赵 阳, 汪承刚, 侯金锋, 袁凌云. 白菜新品种‘皖绿2号’[J]. 园艺学报, 2022, 49(S1): 71-72. |
| [11] | 高彦龙, 吴玉霞, 张仲兴, 王双成, 张瑞, 张德, 王延秀. 苹果ELO家族基因鉴定及其在低温胁迫下的表达分析[J]. 园艺学报, 2022, 49(8): 1621-1636. |
| [12] | 王钰, 张雪, 张学颖, 张思雨, 闻婷婷, 王迎君, 甘彩霞, 庞文星. 抗毒素Camalexin对大白菜抗根肿病的作用研究[J]. 园艺学报, 2022, 49(8): 1689-1698. |
| [13] | 邱子文, 刘林敏, 林永盛, 林晓洁, 李永裕, 吴少华, 杨超. 千层金MbEGS基因的克隆与功能分析[J]. 园艺学报, 2022, 49(8): 1747-1760. |
| [14] | 郑林, 王帅, 刘语诺, 杜美霞, 彭爱红, 何永睿, 陈善春, 邹修平. 柑橘响应黄龙病菌侵染的NAC基因的克隆及表达分析[J]. 园艺学报, 2022, 49(7): 1441-1457. |
| [15] | 张鲁刚, 卢倩倩, 何琼, 薛一花, 马晓敏, 马帅, 聂姗姗, 杨文静. 紫橙色大白菜新种质的创制[J]. 园艺学报, 2022, 49(7): 1582-1588. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司
