
园艺学报 ›› 2021, Vol. 48 ›› Issue (2): 276-288.doi: 10.16420/j.issn.0513-353x.2020-0282
张庆雯, 祁静静, 谢宇, 谢竹, 彭蕴, 李强, 彭爱红, 邹修平, 何永睿, 陈善春*( ), 姚利晓*(
), 姚利晓*( )
)
收稿日期:2020-06-24
修回日期:2020-09-07
出版日期:2021-02-25
发布日期:2021-03-09
通讯作者:
陈善春,姚利晓
E-mail:chenshanchun@cric.cn;yaolixiao@cric.cn
基金资助:
ZHANG Qingwen, QI Jingjing, XIE Yu, XIE Zhu, PENG Yun, LI Qiang, PENG Aihong, ZOU Xiuping, HE Yongrui, CHEN Shanchun*( ), YAO Lixiao*(
), YAO Lixiao*( )
)
Received:2020-06-24
Revised:2020-09-07
Online:2021-02-25
Published:2021-03-09
Contact:
CHEN Shanchun,YAO Lixiao
E-mail:chenshanchun@cric.cn;yaolixiao@cric.cn
摘要:
胼胝质合成酶是控制胼胝质合成的关键酶,在植物生长发育和抗逆胁迫中具有重要作用。克隆并分析‘锦橙’胼胝质合成酶基因CsCalS5及其启动子序列,实时荧光定量PCR检测CsCalS5的组织表达模式以及植物生长调节剂、黄龙病菌和柑橘溃疡病菌的诱导表达模式,并通过组织切片观察感染黄龙病和柑橘溃疡病‘锦橙’叶脉胼胝质的沉积。结果显示,CsCalS5启动子序列中含有脱落酸和病原菌的响应元件;CsCalS5编码1 952个氨基酸含有保守结构域FKS1和β-1,3-葡聚糖合成酶功能域的跨膜蛋白;CsCalS5在‘锦橙’的茎中高表达,且受脱落酸的诱导;黄龙病菌侵染后叶脉韧皮部的胼胝质沉积明显,患病叶片CsCalS5的表达量是健康对照的4.02倍;柑橘溃疡病菌侵染叶片后,叶脉韧皮部胼胝质沉积无明显增加,且CsCalS5的表达量与健康对照无显著差异。综上,黄龙病菌可能通过上调‘锦橙’CsCalS5的表达促进韧皮部胼胝质的沉积,从而增强其防御能力,且这种抗性可能受到脱落酸的调控。
中图分类号:
张庆雯, 祁静静, 谢宇, 谢竹, 彭蕴, 李强, 彭爱红, 邹修平, 何永睿, 陈善春, 姚利晓. 黄龙病菌胁迫下‘锦橙’CsCalS5表达和胼胝质沉积的初步分析[J]. 园艺学报, 2021, 48(2): 276-288.
ZHANG Qingwen, QI Jingjing, XIE Yu, XIE Zhu, PENG Yun, LI Qiang, PENG Aihong, ZOU Xiuping, HE Yongrui, CHEN Shanchun, YAO Lixiao. Preliminary Analysis of CsCalS5 and Callose Deposition in Citrus sinensis Infected with Candidatus Liberibacter asiaticus[J]. Acta Horticulturae Sinica, 2021, 48(2): 276-288.
| 引物名称 Primer name | 引物序列(5′-3′) Primer sequence |
|---|---|
| CsCalS5-1 | F:ATAGAGGCAAAAGATGTCACAGAGA;R:TTTTGCCGATCCATCTGTCAC |
| CsCalS5-2 | F:CGTGCTGACGCTGATTTCT;R:AATAACCTCTTTCTCTCGTTCTCC |
| CsCalS5-3 | F:GCTAGCAAGATACCCATAGCATT;R:CTTAAGGAATTCTTGAAGCAAATTC |
| CsCalS5-4 | F:GGAAACCTGAAAATCAAAACCA;R:TCACTCCTTACTTTTGGAAGACCT |
| CsCalS5P | F:CTCATGCTTTATGCCTTT;R:CTTTTGCCTCTATAAATATGAT |
| Actin | F:CATCCCTCAGCACCTTCC;R:CCAACCTTAGCACTTCTCC |
| qCsCalS5 | F:AACGTTCTGGTGATGCTCGT;R:AGCCTTCGCAAGAGCTGAAT |
| OI1/OI2C | F:GCGCGTATGCAATACGAGCGGCA;R:GCCTCGCGACTTCGCAACCCAT |
表1 本试验中所用引物
Table 1 The sequences of primers in this study
| 引物名称 Primer name | 引物序列(5′-3′) Primer sequence |
|---|---|
| CsCalS5-1 | F:ATAGAGGCAAAAGATGTCACAGAGA;R:TTTTGCCGATCCATCTGTCAC |
| CsCalS5-2 | F:CGTGCTGACGCTGATTTCT;R:AATAACCTCTTTCTCTCGTTCTCC |
| CsCalS5-3 | F:GCTAGCAAGATACCCATAGCATT;R:CTTAAGGAATTCTTGAAGCAAATTC |
| CsCalS5-4 | F:GGAAACCTGAAAATCAAAACCA;R:TCACTCCTTACTTTTGGAAGACCT |
| CsCalS5P | F:CTCATGCTTTATGCCTTT;R:CTTTTGCCTCTATAAATATGAT |
| Actin | F:CATCCCTCAGCACCTTCC;R:CCAACCTTAGCACTTCTCC |
| qCsCalS5 | F:AACGTTCTGGTGATGCTCGT;R:AGCCTTCGCAAGAGCTGAAT |
| OI1/OI2C | F:GCGCGTATGCAATACGAGCGGCA;R:GCCTCGCGACTTCGCAACCCAT |
图1 CsCalS5的4个片段及其启动子的PCR扩增 M:Marker;1 ~ 4:CsCalS5-1,2,3,4的PCR扩增产物;5P:启动子序列;CK+:阳性对照;CK-:阴性对照。
Fig. 1 PCR amplifications of four fragments and promoter of CsCalS5 M:Marker;1-4:PCR product of CsCalS5-1,2,3,4;5P:Promoter sequence;CK+:Positive control;CK-:Negative control.
| 元件名称 Element name | 序列 Sequence | 数量 Number | 功能 Function | 位置 Location |
|---|---|---|---|---|
| GT1 box | GAAAAA | 2 | 病原菌和盐响应 Pathogen or salt-induced responsiveness | -264(+)/-976(-) |
| dof box | AAAG | 23 | 病原菌响应Pathogen responsiveness | -1281/-1178/-1097/-974/-927/ -838/-260/-161/-148/-81/-7(+)-1486/-1340/-1264/-1060/-869/-707/-656/-610/-545/-479/-207/-85(-) |
| W box | TGACT | 2 | 病原菌和损伤响应 Pathogen and wound responsiveness | -474(+)/-582(+) |
| GARE-motif | TCTGTTG | 2 | 赤霉素响应元件Gibberellin-responsive element | -281(+) |
| CCTTTT | -1490(+) | |||
| TCA-element | CCATCTTTTT | 1 | 水杨酸响应元件Salicylic acid responsiveness | -1067(+) |
| ABRE | ACGTG | 1 | 脱落酸响应元件Abscisic acid responsiveness | -814(+) |
| WUN-motif | AAATTACTA | 1 | 损伤响应元件Wound responsiveness | -1369(-) |
| MYC | CATTTG | 2 | 干旱和冻害响应元件 Drought and freeze responsiveness | -1473(+)/-1400(-) |
| MBS | CAACTG | 1 | 参与干旱诱导Involved in drought-inducibility | -1259(-) |
| ARE | AAACCA | 2 | 厌氧诱导所需Essential for the anaerobic induction | -1255(+)/-456(-) |
| MBSI | TTTTTACGGTTA | 1 | 参与类黄酮合成基因的调控 Involved in flavonoid biosynthetic genes regulation | -131(+) |
| RY-element | CATGCATG | 1 | 参与种子特异性调控 Involved in seed-specific regulation | -696(-) |
| GATA-motif | AAGATAAGATT | 1 | 光响应元件Light responsiveness | -1400(-) |
| GT1-motif | GGTTAA | 1 | 光响应元件Light responsiveness | -1336(-) |
| chs-CMA2a | TCACTTGA | 1 | 光响应元件Light responsiveness | -1268(-) |
| Box 4 | ATTAAT | 1 | 光响应元件Light responsiveness | -1293(+) |
| LAMP-element | CTTTATCA | 1 | 光响应元件Light responsiveness | -1123(+) |
| G-box | TACGTG | 2 | 光响应元件Light responsiveness | -810(+) |
| GGTTAAT | -194(+) |
表2 CsCalS5启动子的顺式作用元件
Table 2 cis-Acting elements of CsCalS5 promoter
| 元件名称 Element name | 序列 Sequence | 数量 Number | 功能 Function | 位置 Location |
|---|---|---|---|---|
| GT1 box | GAAAAA | 2 | 病原菌和盐响应 Pathogen or salt-induced responsiveness | -264(+)/-976(-) |
| dof box | AAAG | 23 | 病原菌响应Pathogen responsiveness | -1281/-1178/-1097/-974/-927/ -838/-260/-161/-148/-81/-7(+)-1486/-1340/-1264/-1060/-869/-707/-656/-610/-545/-479/-207/-85(-) |
| W box | TGACT | 2 | 病原菌和损伤响应 Pathogen and wound responsiveness | -474(+)/-582(+) |
| GARE-motif | TCTGTTG | 2 | 赤霉素响应元件Gibberellin-responsive element | -281(+) |
| CCTTTT | -1490(+) | |||
| TCA-element | CCATCTTTTT | 1 | 水杨酸响应元件Salicylic acid responsiveness | -1067(+) |
| ABRE | ACGTG | 1 | 脱落酸响应元件Abscisic acid responsiveness | -814(+) |
| WUN-motif | AAATTACTA | 1 | 损伤响应元件Wound responsiveness | -1369(-) |
| MYC | CATTTG | 2 | 干旱和冻害响应元件 Drought and freeze responsiveness | -1473(+)/-1400(-) |
| MBS | CAACTG | 1 | 参与干旱诱导Involved in drought-inducibility | -1259(-) |
| ARE | AAACCA | 2 | 厌氧诱导所需Essential for the anaerobic induction | -1255(+)/-456(-) |
| MBSI | TTTTTACGGTTA | 1 | 参与类黄酮合成基因的调控 Involved in flavonoid biosynthetic genes regulation | -131(+) |
| RY-element | CATGCATG | 1 | 参与种子特异性调控 Involved in seed-specific regulation | -696(-) |
| GATA-motif | AAGATAAGATT | 1 | 光响应元件Light responsiveness | -1400(-) |
| GT1-motif | GGTTAA | 1 | 光响应元件Light responsiveness | -1336(-) |
| chs-CMA2a | TCACTTGA | 1 | 光响应元件Light responsiveness | -1268(-) |
| Box 4 | ATTAAT | 1 | 光响应元件Light responsiveness | -1293(+) |
| LAMP-element | CTTTATCA | 1 | 光响应元件Light responsiveness | -1123(+) |
| G-box | TACGTG | 2 | 光响应元件Light responsiveness | -810(+) |
| GGTTAAT | -194(+) |

图6 健康(A、C)和感染黄龙病(B、D)‘锦橙’叶脉 黄色箭头指向韧皮部塌陷,红色箭头指向的蓝色荧光为胼胝质沉积。Co:皮层;P:韧皮部;Fi:纤维;Pi:髓;X:木质部。
Fig. 6 Vein of healthy(A,C)and CLas-infected(B,D)C. sinensis Yellow arrows point to the collapse of the phloem,red arrows point to the callose with blue fluorescence. Co:Cortex;P:Phloem;Fi:Fiber;Pi:Pith;X:Xylem.
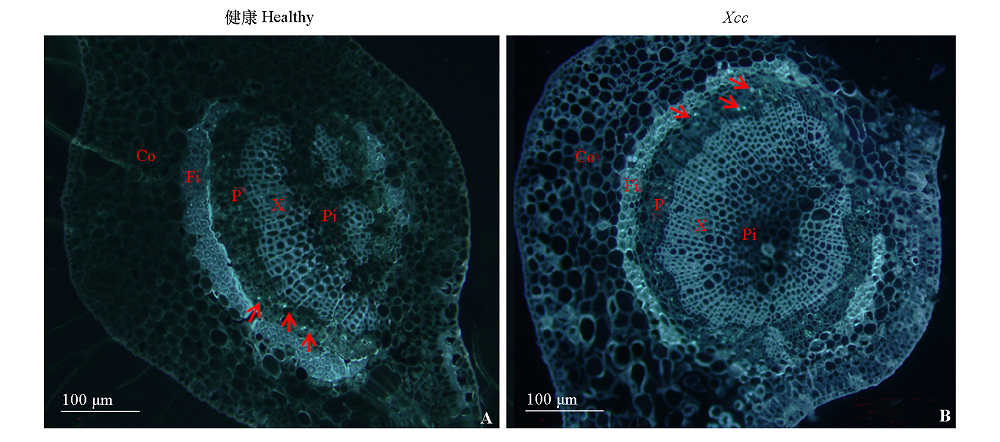
图8 健康‘锦橙’(A)和感染溃疡病‘锦橙’(B)叶脉 红色箭头指向的蓝色荧光为胼胝质沉积。Co:皮层;P:韧皮部;Fi:纤维;Pi:髓;X:木质部。
Fig. 8 Vein of healthy(A)and Xcc-infected(B)C. sinensis Red arrows point to the callose with blue fluorescence. Co:Cortex;P:Phloem;Fi:Fiber;Pi:Pith;X:Xylem.
| [1] |
Albrecht U, Bowman K D. 2008. Gene expression in Citrus sinensis(L.)Osbeck following infection with the bacterial pathogen Candidatus Liberibacter asiaticus causing Huanglongbing in Florida. Plant Science, 175:291-306.
doi: 10.1016/j.plantsci.2008.05.001 URL |
| [2] |
Boava L P, Cristofani-Yaly M, Machado M A. 2017. Physiologic,anatomic and gene expression changes in Citrus sunki,Poncirus trifoliata and their hybrids after‘Candidatus Liberibacter asiaticus’infection. Phytopathology, 107:590-599.
doi: 10.1094/PHYTO-02-16-0077-R URL |
| [3] | Chen X Y, Kim J Y. 2009. Callose synthesis in higher plants. Plant Signaling & Behavior, 4:489-492. |
| [4] |
Chen Y, Yu P, Luo J, Jiang Y. 2003. Secreted protein prediction system combining CJ-SPHMM,TMHMM,and PSORT. Mammalian Genome, 14:859-865.
doi: 10.1007/s00335-003-2296-6 URL |
| [5] |
Cui W, Lee J Y. 2016. Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nature Plants, 2:16034.
doi: 10.1038/nplants.2016.34 URL |
| [6] | Dong Cui-cui, Ma Yan-yan, Xie Rang-jin, Deng Lie, Yi Shi-lai, Lü Qiang, Zheng Yong-qiang, He Shao-lan. 2016. Expression of two Citrus AP2/ERF genes under different hormone and stress treatments. Acta Horticulturae Sinica, 43(2):41-50. (in Chinese) |
| 董翠翠, 马岩岩, 谢让金, 邓烈, 易时来, 吕强, 郑永强, 何绍兰. 2016. 柑橘CitEFR9和CitAP2-7在不同逆境和外源激素处理下的表达. 园艺学报, 43(2):41-50. | |
| [7] |
Dong X, Hong Z, Chatterjee J, Kim S, Verma D P S. 2008. Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta, 229:87-98.
doi: 10.1007/s00425-008-0812-3 URL |
| [8] |
Enrique R, Siciliano F, Favaro M A, Gerhardt N, Roeschlin R, Rigano L, Sendin L, Castagnaro A, Vojnov A, Marano M R. 2011. Novel demonstration of RNAi in citrus reveals importance of citrus callose synthase in defence against Xanthomonas citri subsp. citri. Plant Biotechnology Journal, 9:394-407.
doi: 10.1111/j.1467-7652.2010.00555.x pmid: 20809929 |
| [9] |
Ellinger D, Naumann M, Falter C, Zwikowics C, Jamrow T, Manisseri C, Somerville S C, Voigt C A. 2013. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiology, 161:1433-1444.
doi: 10.1104/pp.112.211011 URL |
| [10] | Estrella L, Victoria P, Jérôme R, Flors V, Mauch-Mani B, Ton J. 2011. Callose deposition:a multifaceted plant defense response. Molecular Plant-microbe Interaction, 24:183-193. |
| [11] |
Fan J, Chen C, Yu Q, Khalaf A, Achor D S, Brlansky R H, Moore G A, Li Z G, Gmitter F G. 2012. Comparative transcriptional and anatomical analyses of tolerant rough lemon and susceptible sweet orange in response to‘Candidatus Liberibacter asiaticus’infection. Mol Plant Microbe Interact, 25:1396-407.
doi: 10.1094/MPMI-06-12-0150-R URL |
| [12] |
Finn R D, Tate J, Mistry J, Tate J, Coggill P, Heger A, Pollington J E, Gavin O L, Gunasekaran P, Ceric G, Forslund K, Holm L, Sonnhammer E, Eddy S R, Bateman A. 2008. The pfam protein families database. Nucleic Acids Research, 32:D138-41.
doi: 10.1093/nar/gkh121 URL |
| [13] |
Fromm J R, Hajirezaei M R, Becker V K, Lautner S. 2013. Electrical signaling along the phloem and its physiological responses in the maize leaf. Frontiers in Plant Science, 4:239.
doi: 10.3389/fpls.2013.00239 pmid: 23847642 |
| [14] |
Fu M, Xu M, Zhou T, Wang D, Tian S, Han L, Dong H, Zhang C. 2014. Transgenic expression of a functional fragment of harpin protein Hpa1 in wheat induces the phloem-based defence against English grain aphid. Journal of Experimental Botany, 65:1439-1453.
doi: 10.1093/jxb/ert488 URL |
| [15] |
Gamir J, Pastor V, Sánchez-Bel P, Agut B, Mateu D, García-Andrade J, Flors V. 2018. Starch degradation,abscisic acid and vesicular trafficking are important elements in callose priming by indole-3-carboxylic acid in response to Plectosphaerella cucumerina infection. The Plant Journal, 96:518-531.
doi: 10.1111/tpj.14045 URL |
| [16] | Geourjon C, Deléage G. 1996. SOPMA:Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Computer Applications in the Biosciences Cabios, 11:681-684. |
| [17] |
Granato L M, Galdeano D M, D’Alessandre N D R, Breton M C, Machado M A. 2019. Callose synthase family genes plays an important role in the Citrus defense response to Candidatus Liberibacter asiaticus. European Journal of Plant Pathology, 155:25-38
doi: 10.1007/s10658-019-01747-6 URL |
| [18] |
Gurr S J, Rushton P J. 2005. Engineering plants with increased disease resistance:How are we going to express it? Trends in Biotechnology, 23:283-290.
doi: 10.1016/j.tibtech.2005.04.009 URL |
| [19] |
Higo K, Ugawa Y, Iwamoto M, Korenaga T. 1999. Plant cis-acting regulatory DNA elements(PLACE)database:1999. Nucleic acids Research, 27:297-300.
doi: 10.1093/nar/27.1.297 URL |
| [20] | Hocquellet A, Toorawa P, Bové J M, Garnier M. 1999. Detection and identification of the two Candidatus Liberobacter species associated with citrus Huanglongbing by PCR amplification of ribosomal protein genes of the beta operon. Molecular & Cellular probes, 13(5):373-379. |
| [21] |
Hong Z, Delauney A J, Verma D P. 2001. A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell, 13:755-768.
pmid: 11283334 |
| [22] |
Kumar S, Stecher G, Tamura K. 2016. MEGA7:molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33:1870-1874.
doi: 10.1093/molbev/msw054 URL |
| [23] | Jia Rui-rui. 2018. Gene cloning and expression analysis of canker-related transcription factor CsBZIP40 in citrus[M. D. Dissertation]. Chongqing:Southwest University. (in Chinese). |
| 贾瑞瑞. 2018. 柑橘溃疡病相关转录因子CsBZIP40的功能研究[硕士论文]. 重庆:西南大学. | |
| [24] |
Lescot M. 2002. PlantCARE,a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research, 30:325-327.
doi: 10.1093/nar/30.1.325 URL |
| [25] |
Liu J, Du H, Ding X, Zhou Y, Xie P, Wu J. 2017. Mechanisms of callose deposition in rice regulated by exogenous abscisic acid and its involvement in rice resistance to Nilaparvata lugens Stal(Hemiptera︰Delphacidae). Pest Management Science, 73:2559-2568.
doi: 10.1002/ps.2017.73.issue-12 URL |
| [26] | Long Q, Xie Y, He Y, Li Q, Zou X, Chen S. 2019. Abscisic acid promotes jasmonic acid accumulation and plays a key role in citrus canker development. Frontiers in Plant Science. 10:1634. |
| [27] |
Lü B, Sun W, Zhang S, Zhang C, Qian J, Wang X, Gao R, Dong H. 2011. HrpNEa-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana. Journal of Biosciences, 36:123-137.
doi: 10.1007/s12038-011-9016-2 URL |
| [28] |
Nishimura M T, Stein M, Hou B H, Vogel J P, Edwards H, Somerville S C. 2003. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science, 301:969-972.
doi: 10.1126/science.1086716 URL |
| [29] |
Mauch-Mani B, Baccelli I, Luna E, Flors V. 2017. Defense Priming:An Adaptive Part of Induced Resistance. Annual Review of Plant Biology, 68:485-512.
doi: 10.1146/annurev-arplant-042916-041132 pmid: 28226238 |
| [30] |
Nedukha O M. 2015. Callose:Localization,functions,and synthesis in plant cells. Cytology and Genetics, 49:49-57.
doi: 10.3103/S0095452715010090 URL |
| [31] |
O'Lexy R, Kasai K, Clark N, Fujiwara T, Sozzani R, Gallagher K L. 2018. Exposure to heavy metal stress triggers changes in plasmodesmatal permeability via deposition and breakdown of callose. Journal of Experimental Botany, 69:3715-3728.
doi: 10.1093/jxb/ery171 URL |
| [32] |
Oide S, Bejai S, Staal J, Guan N, Kaliff M, Dixelius C. 2013. A novel role of PR2 in abscisic acid(ABA)mediated,pathogen-induced callose deposition in Arabidopsis thaliana. New Phytologist, 200:1187-1199.
doi: 10.1111/nph.2013.200.issue-4 URL |
| [33] | Panu A, Manohar J, Konstantin A, Delphine B, Gabor C, Edouard C, Séverine D, Volker F, Arnaud F, Elisabeth G. 2012. ExPASy:SIB bioinformatics resource portal. Nucleic Acids 40 (Web Server issue):W597-603 |
| [34] | Peng Yun, Fan Hai-fang, Lei Tian-gang, He Yong-rui, Chen Shan-chun, Yao Li-xiao. 2019. Expression analysis of callose synthase gene family in citrus. Acta Horticulturae Sinica, 46(2):330-336. (in Chinese) |
| 彭蕴, 范海芳, 雷天刚, 何永睿, 陈善春, 姚利晓. 2019. 柑橘胼胝质合成酶基因家族的表达分析. 园艺学报, 46(2):330-336. | |
| [35] |
Pramod S, Thomas V, Rao K S, Krishnakumar R. 2011. Definitive callose deposition in tapping panel dryness affected bark of Hevea brasiliensis. Journal of Sustainable Forestry, 30:329-342.
doi: 10.1080/10549811.2011.532032 URL |
| [36] |
Park H C, Kim M L, Kang Y H, Jeon J M, Yoo J H, Kim M C, Park C Y, Jeong J C, Moon B C, Lee J H, Yoon H W, Lee S H, Chung W S, Lim C O, Lee S Y, Hong J C, Cho M J. 2004. Pathogen- and NaCl-induced expression of the SCaM-4 promoter Is mediated in part by a GT-1 Box that interacts with a GT-1-Like transcription factor1. Plant Physiology, 135:2150-2161.
doi: 10.1104/pp.104.041442 URL |
| [37] |
Saatian B, Austin R S, Tian G, Chen C, Vi N, Kohalmi S E, Geelen D, Cui Y. 2018. Analysis of a novel mutant allele of GSL8 reveals its key roles in cytokinesis and symplastic trafficking in Arabidopsis. BMC Plant Biology, 18:295.
doi: 10.1186/s12870-018-1515-y pmid: 30466394 |
| [38] |
Shimwela M M, Narouei-Khandan H A, Halbert S E, Keremane M L, Minsavage G V, Timilsina S, Massawe D P, Jones J B, van Bruggen A H C. 2016. First occurrence of Diaphorina citriin East Africa,characterization of the Ca. Liberibacter species causing Huanglongbing(HLB)in Tanzania,and potential further spread of D. citriand HLB in Africa and Europe. European Journal of Plant Pathology, 146:349-368.
doi: 10.1007/s10658-016-0921-y URL |
| [39] | Valente A, Diann A, Frederick G G, Gene A, Nian W. 2013. Transcriptional and microscopic analyses of citrus stem and root responses to Candidatus Liberibacter asiaticus Infection. PlLS NE, 8:e73742. |
| [40] |
Wang X, Xu Y, Zhang S Q, Cao L, Huang Y, Cheng J F, Wu G Z, Tian S L, Chen C L, Liu Y, Yu H W, Yang X M, Lan H, Wang N, Wang L, Xu J D, Jiang X L, Xie Z Z, Tan M L, Larkin R M, Chen L L, Ma B G, Ruan Y J, Deng X X, Xu Q. 2017. Genomic analyses of primitive,wild and cultivated citrus provide insights into asexual reproduction. Nature Genetic, 49:765-772.
doi: 10.1038/ng.3839 URL |
| [41] |
Wang Z, Li X, Wang X, Liu N, Xu B, Peng Q, Guo Z, Fan B, Zhu C, Chen Z. 2019. Arabidopsis endoplasmic reticulum-localized UBAC2 proteins interact with PAMP-INDUCED COILED-COIL to regulate pathogen-induced callose deposition and plant immunity. Plant Cell, 31:153-171.
doi: 10.1105/tpc.18.00334 |
| [42] |
Wawrzynska A, Rodibaugh N L, Innes R W. 2010. Synergistic activation of defense responses in Arabidopsis by simultaneous loss of the GSL5 callose synthase and the EDR1 protein kinase. Mol Plant Microbe Interact, 23:578-584.
doi: 10.1094/MPMI-23-5-0578 URL |
| [43] | Wen Qingli, Xie Zhu, Wu Liu, He Yongrui, Chen Shanchun, Zou Xiuping. 2018. Clone and expression analysis of the Citrus Phloem Protein 2 gene CsPP2B15 responding to Huanglongbing infection in citrus. Acta Horticulturae Sinica, 45(12):2347-2357. (in Chinese) |
| 文庆利, 谢竹, 吴柳, 何永睿, 陈善春, 邹修平. 2018. 柑橘响应黄龙病侵染的韧皮部蛋白2基因CsPP2B15的克隆与表达分析. 园艺学报, 2018,45(12):2347-2357. | |
| [44] | Xie B, Wang X, Zhu M, Zhang Z, Hong Z. 2011. CalS7 encodes a callose synthase responsible for callose deposition in the phloem. Plant Journal for Cell & Molecular Biology, 65:1-14. |
| [45] | Xie Zhu, Zhao Ke, Zheng Lin, Long Junhong, Du Meixia, He Yongrui, Chen Shanchun, Zou Xiuping. 2020. Cloning and expression analysis of alcohol dehydrogenase CsADH1 gene responding to Huanglongbing infection in Citrus. Acta Horticulturae Sinica, 47(3):445-454. (in Chinese) |
| 谢竹, 赵珂, 郑林, 龙俊宏, 杜美霞, 何永睿, 陈善春, 邹修平. 2020. 响应黄龙病侵染的柑橘乙醇脱氢酶基因CsADH1的克隆与表达分析. 园艺学报, 47(3):445-454. | |
| [46] |
Xu Q, Chen L L, Ruan X, Chen D, Zhu A, Chen C, Bertrand D, Jiao W B, Hao B H, Lyon M P, Chen J, Gao S, Xing F, Lan H, Chang J W, Ge X, Lei Y, Hu Q, Miao Y, Wang L, Xiao S, Biswas M K, Zeng W, Guo F, Cao H, Yang X, Xu X W, Cheng Y J, Xu J, Liu J H, Luo O J, Tang Z, Guo W W, Kuang H, Zhang H Y, Roose M L, Nagarajan N, Deng X X, Ruan Y. 2013. The draft genome of sweet orange(Citrus sinensis). Nature Genetics, 45:59-66.
doi: 10.1038/ng.2472 URL |
| [47] |
Yu J, Zhang Y, Di C, Zhang Q, Zhang K, Wang C, You Q, Yan H, Dai S Y, Yuan J S. 2015. JAZ7 negatively regulates dark-induced leaf senescence in Arabidopsis. Journal of Experimental Botany, 67:751-762.
doi: 10.1093/jxb/erv487 URL |
| [48] | Zhang H, Shi W L, You J F, Bian M D, Qin X M, Hui Y U, Liu Q, Ryan P R, Yang Z M. 2015. Transgenic Arabidopsis thaliana plants expressing a β-1,3-glucanase from sweet sorghum(Sorghum bicolorβL.)show reduced callose deposition and increased tolerance to aluminium toxicity. Plant Cell & Environment, 38:1178-1188. |
| [49] |
Zhu C Q, Zheng X J, Huang Y, Ye J L, Chen P, Zhang C L, Zhao F, Xie Z Z, Zhang S Q, Wang N, Li H, Wang L, Tang X M, Chai L J, Xu Q, Deng X X. 2019. Genome sequencing and CRISPR/Cas9 gene editing of an early flowering mini citrus(Fortunella hindsii). Plant Biotechnology Journal, 17:2199-2210.
doi: 10.1111/pbi.v17.11 URL |
| [50] | Zhang Y Y, Liu X L, Huang H H. 2012. Molecular cloning of Crustin-like gene in the white shrimp(Litopenaeus vannamei)and its mRNA expression with Vibrio parahaemolyticus challenge. Journal of Northwest A & F University, 40:119-132. |
| [51] | Zhong Xi. 2018. Transcriptomic and proteomic analysis of Citrus hystrix responses to‘Candidatus Liberibacter asiaticus’in early and late stage of infection[M. D. Dissertation]. Chongqing:Southwest University. (in Chinese) |
| 钟晰. 2018. 马蜂柑响应黄龙病菌侵染前期与后期的转录组和蛋白组学研究[硕士论文]. 重庆:西南大学. |
| [1] | 叶子茂, 申晚霞, 刘梦雨, 王 彤, 张晓楠, 余 歆, 刘小丰, 赵晓春, . R2R3-MYB转录因子CitMYB21对柑橘类黄酮生物合成的影响[J]. 园艺学报, 2023, 50(2): 250-264. |
| [2] | 蒋靖东, 韦壮敏, 王楠, 朱晨桥, 叶俊丽, 谢宗周, 邓秀新, 柴利军. 山金柑四倍体资源的发掘与鉴定[J]. 园艺学报, 2023, 50(1): 27-35. |
| [3] | 杜玉玲, 杨凡, 赵娟, 刘书琪, 龙超安. 新鱼腥草素钠对柑橘指状青霉的抑菌作用[J]. 园艺学报, 2023, 50(1): 145-152. |
| [4] | 赵雪艳, 王琪, 王莉, 王方圆, 王庆, 李艳. 基于比较转录组的延胡索组织差异性表达分析[J]. 园艺学报, 2023, 50(1): 177-187. |
| [5] | 李镇希, 潘睿翾, 许美容, 郑正, 邓晓玲. 柑橘黄龙病菌双重实时荧光PCR检测方法的建立[J]. 园艺学报, 2023, 50(1): 188-196. |
| [6] | 朱凯杰, 张哲惠, 曹立新, 向舜德, 叶俊丽, 谢宗周, 柴利军, 邓秀新, . 棕色晚熟脐橙新品种‘宗橙’[J]. 园艺学报, 2022, 49(S1): 41-42. |
| [7] | 朱世平, 文荣中, 王媛媛, 曾 杨. 特晚熟柑橘新品种‘金乐柑’[J]. 园艺学报, 2022, 49(S1): 43-44. |
| [8] | 高彦龙, 吴玉霞, 张仲兴, 王双成, 张瑞, 张德, 王延秀. 苹果ELO家族基因鉴定及其在低温胁迫下的表达分析[J]. 园艺学报, 2022, 49(8): 1621-1636. |
| [9] | 邱子文, 刘林敏, 林永盛, 林晓洁, 李永裕, 吴少华, 杨超. 千层金MbEGS基因的克隆与功能分析[J]. 园艺学报, 2022, 49(8): 1747-1760. |
| [10] | 郑林, 王帅, 刘语诺, 杜美霞, 彭爱红, 何永睿, 陈善春, 邹修平. 柑橘响应黄龙病菌侵染的NAC基因的克隆及表达分析[J]. 园艺学报, 2022, 49(7): 1441-1457. |
| [11] | 杨海健, 张云贵, 周心智. 柑橘新品种‘云贵脆橙’[J]. 园艺学报, 2022, 49(7): 1611-1612. |
| [12] | 马维峰, 李艳梅, 马宗桓, 陈佰鸿, 毛娟. 苹果POD家族基因的鉴定与MdPOD15的功能分析[J]. 园艺学报, 2022, 49(6): 1181-1199. |
| [13] | 张凯, 麻明英, 王萍, 李益, 金燕, 盛玲, 邓子牛, 马先锋. 柑橘HSP20家族基因鉴定及其响应溃疡病菌侵染表达分析[J]. 园艺学报, 2022, 49(6): 1213-1232. |
| [14] | 李文婷, 李翠晓, 林小清, 郑永钦, 郑正, 邓晓玲. 基于STR位点对广东省柑橘溃疡病菌种群遗传结构的分析[J]. 园艺学报, 2022, 49(6): 1233-1246. |
| [15] | 麻明英, 郝晨星, 张凯, 肖桂华, 苏翰英, 文康, 邓子牛, 马先锋. 甜橙SWEET2a促进柑橘溃疡病菌侵染[J]. 园艺学报, 2022, 49(6): 1247-1260. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司